The metalloprotease of Listeria monocytogenes is regulated by pH
- PMID: 21803995
- PMCID: PMC3187447
- DOI: 10.1128/JB.05134-11
The metalloprotease of Listeria monocytogenes is regulated by pH
Abstract
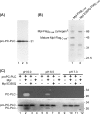
Listeria monocytogenes is an intracytosolic bacterial pathogen. Among the factors contributing to escape from vacuoles are a phosphatidylcholine phospholipase C (PC-PLC) and a metalloprotease (Mpl). Both enzymes are translocated across the bacterial membrane as inactive proproteins, whose propeptides serve in part to maintain them in association with the bacterium. We have shown that PC-PLC maturation is regulated by Mpl and pH and that Mpl maturation occurs by autocatalysis. In this study, we tested the hypothesis that Mpl activity is pH regulated. To synchronize the effect of pH on bacteria, the cytosolic pH of infected cells was manipulated immediately after radiolabeling de novo-synthesized bacterial proteins. Immunoprecipitation of secreted Mpl from host cell lysates revealed the presence of the propeptide and catalytic domain in samples treated at pH 6.5 but not at pH 7.3. The zymogen was present in small amounts under all conditions. Since proteases often remain associated with their respective propeptide following autocatalysis, we aimed at determining whether pH regulates autocatalysis or secretion of the processed enzyme. For this purpose, we used an Mpl construct that contains a Flag tag at the N terminus of its catalytic domain and antibodies that can distinguish N-terminal and non-N-terminal Flag. By fluorescence microscopy, we observed the Mpl zymogen associated with the bacterium at physiological pH but not following acidification. Mature Mpl was not detected in association with the bacterium at either pH. Using purified proteins, we determined that processing of the PC-PLC propeptide by mature Mpl is also pH sensitive. These results indicate that pH regulates the activity of Mpl on itself and on PC-PLC.
Figures





Similar articles
-
Differentiation of propeptide residues regulating the compartmentalization, maturation and activity of the broad-range phospholipase C of Listeria monocytogenes.Biochem J. 2010 Dec 15;432(3):557-63. doi: 10.1042/BJ20100557. Biochem J. 2010. PMID: 20879990 Free PMC article.
-
The propeptide of the metalloprotease of Listeria monocytogenes controls compartmentalization of the zymogen during intracellular infection.J Bacteriol. 2009 Jun;191(11):3594-603. doi: 10.1128/JB.01168-08. Epub 2009 Apr 3. J Bacteriol. 2009. PMID: 19346305 Free PMC article.
-
The metalloprotease of Listeria monocytogenes controls cell wall translocation of the broad-range phospholipase C.J Bacteriol. 2005 Apr;187(8):2601-8. doi: 10.1128/JB.187.8.2601-2608.2005. J Bacteriol. 2005. PMID: 15805506 Free PMC article.
-
Bacterial phospholipases and intracellular growth: the two distinct phospholipases C of Listeria monocytogenes.Symp Ser Soc Appl Microbiol. 1998;27:7S-14S. doi: 10.1046/j.1365-2672.1998.0840s107s.x. Symp Ser Soc Appl Microbiol. 1998. PMID: 9750357 Review. No abstract available.
-
Extracellular metalloproteases from bacteria.Appl Microbiol Biotechnol. 2011 Oct;92(2):253-62. doi: 10.1007/s00253-011-3532-8. Epub 2011 Aug 16. Appl Microbiol Biotechnol. 2011. PMID: 21845384 Review.
Cited by
-
An M29 Aminopeptidase from Listeria Monocytogenes Contributes to In Vitro Bacterial Growth but not to Intracellular Infection.Microorganisms. 2020 Jan 13;8(1):110. doi: 10.3390/microorganisms8010110. Microorganisms. 2020. PMID: 31941013 Free PMC article.
-
A non-catalytic histidine residue influences the function of the metalloprotease of Listeria monocytogenes.Microbiology (Reading). 2014 Jan;160(Pt 1):142-148. doi: 10.1099/mic.0.071779-0. Epub 2013 Oct 18. Microbiology (Reading). 2014. PMID: 24140648 Free PMC article.
-
Posttranslocation chaperone PrsA2 regulates the maturation and secretion of Listeria monocytogenes proprotein virulence factors.J Bacteriol. 2011 Nov;193(21):5961-70. doi: 10.1128/JB.05307-11. Epub 2011 Sep 9. J Bacteriol. 2011. PMID: 21908675 Free PMC article.
-
Protein transport across the cell wall of monoderm Gram-positive bacteria.Mol Microbiol. 2012 May;84(3):405-13. doi: 10.1111/j.1365-2958.2012.08040.x. Epub 2012 Apr 4. Mol Microbiol. 2012. PMID: 22471582 Free PMC article. Review.
-
The Metalloprotease Mpl Supports Listeria monocytogenes Dissemination through Resolution of Membrane Protrusions into Vacuoles.Infect Immun. 2016 May 24;84(6):1806-1814. doi: 10.1128/IAI.00130-16. Print 2016 Jun. Infect Immun. 2016. PMID: 27068088 Free PMC article.
References
-
- Bishop D. K., Hinrichs D. J. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005–2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

