Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling
- PMID: 21804089
- PMCID: PMC3171937
- DOI: 10.1681/ASN.2010101086
Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling
Abstract
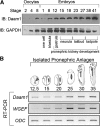
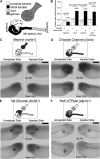
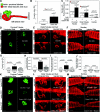
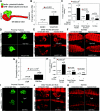
Canonical β-catenin-mediated Wnt signaling is essential for the induction of nephron development. Noncanonical Wnt/planar cell polarity (PCP) pathways contribute to processes such as cell polarization and cytoskeletal modulation in several tissues. Although PCP components likely establish the plane of polarization in kidney tubulogenesis, whether PCP effectors directly modulate the actin cytoskeleton in tubulogenesis is unknown. Here, we investigated the roles of Wnt PCP components in cytoskeletal assembly during kidney tubule morphogenesis in Xenopus laevis and zebrafish. We found that during tubulogenesis, the developing pronephric anlagen expresses Daam1 and its interacting Rho-GEF (WGEF), which compose one PCP/noncanonical Wnt pathway branch. Knockdown of Daam1 resulted in reduced expression of late pronephric epithelial markers with no apparent effect upon early markers of patterning and determination. Inhibiting various points in the Daam1 signaling pathway significantly reduced pronephric tubulogenesis. These data indicate that pronephric tubulogenesis requires the Daam1/WGEF/Rho PCP pathway.
Figures

Comment in
-
Making a tubule the noncanonical way.J Am Soc Nephrol. 2011 Sep;22(9):1575-7. doi: 10.1681/ASN.2011070710. Epub 2011 Aug 11. J Am Soc Nephrol. 2011. PMID: 21836144 No abstract available.
References
-
- Park JS, Valerius MT, McMahon AP: Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development 134: 2533–2539, 2007 - PubMed
-
- Shu W, Jiang YQ, Lu MM, Morrisey EE: Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 129: 4831–4842, 2002 - PubMed
-
- De Langhe SP, Sala FG, Del Moral PM, Fairbanks TJ, Yamada KM, Warburton D, Burns RC, Bellusci S: Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol 277: 316–331, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

