A critical period for auditory thalamocortical connectivity
- PMID: 21804538
- PMCID: PMC3419581
- DOI: 10.1038/nn.2882
A critical period for auditory thalamocortical connectivity
Abstract
Neural circuits are shaped by experience during periods of heightened brain plasticity in early postnatal life. Exposure to acoustic features produces age-dependent changes through largely unresolved cellular mechanisms and sites of origin. We isolated the refinement of auditory thalamocortical connectivity by in vivo recordings and day-by-day voltage-sensitive dye imaging in an acute brain slice preparation. Passive tone-rearing modified response strength and topography in mouse primary auditory cortex (A1) during a brief, 3-d window, but did not alter tonotopic maps in the thalamus. Gene-targeted deletion of a forebrain-specific cell-adhesion molecule (Icam5) accelerated plasticity in this critical period. Consistent with its normal role of slowing spinogenesis, loss of Icam5 induced precocious stubby spine maturation on pyramidal cell dendrites in neocortical layer 4 (L4), identifying a primary locus of change for the tonotopic plasticity. The evolving postnatal connectivity between thalamus and cortex in the days following hearing onset may therefore determine a critical period for auditory processing.
Figures


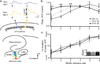




References
-
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. - PubMed
-
- Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–121. - PubMed
-
- Werker JF, Tees RC. Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Dev Psychobiol. 2005;46:233–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

