The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line
- PMID: 21804562
- PMCID: PMC3164356
- DOI: 10.1038/nbt.1932
The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line
Abstract
Chinese hamster ovary (CHO)-derived cell lines are the preferred host cells for the production of therapeutic proteins. Here we present a draft genomic sequence of the CHO-K1 ancestral cell line. The assembly comprises 2.45 Gb of genomic sequence, with 24,383 predicted genes. We associate most of the assembled scaffolds with 21 chromosomes isolated by microfluidics to identify chromosomal locations of genes. Furthermore, we investigate genes involved in glycosylation, which affect therapeutic protein quality, and viral susceptibility genes, which are relevant to cell engineering and regulatory concerns. Homologs of most human glycosylation-associated genes are present in the CHO-K1 genome, although 141 of these homologs are not expressed under exponential growth conditions. Many important viral entry genes are also present in the genome but not expressed, which may explain the unusual viral resistance property of CHO cell lines. We discuss how the availability of this genome sequence may facilitate genome-scale science for the optimization of biopharmaceutical protein production.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
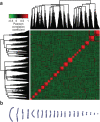

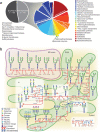


Comment in
-
First CHO genome.Nat Biotechnol. 2011 Aug 5;29(8):718-20. doi: 10.1038/nbt.1943. Nat Biotechnol. 2011. PMID: 21822249 No abstract available.
References
-
- Walsh G. Biopharmaceutical benchmarks 2010. Nat. Biotechnol. 2010;28:917–924. - PubMed
-
- Lim Y, et al. Engineering mammalian cells in bioprocessing—current achievements and future perspectives. Biotechnol. Appl. Biochem. 2010;55:175–189. - PubMed
-
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004;22:1393–1398. - PubMed
-
- Seth G, Charaniya S, Wlaschin KF, Hu WS. In pursuit of a super producer-alternative paths to high producing recombinant mammalian cells. Curr. Opin. Biotechnol. 2007;18:557–564. - PubMed
-
- Derouazi, M. et al. Stability and cytogenetic characterization of recombinant CHO cell lines established by microinjection and phosphate transfection. in Cell Technology for Cell Products (ed. Smith, R.) 443–446 (Springer Netherlands, 2007).
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

