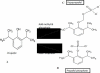
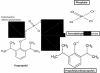
Fospropofol: clinical pharmacology
Figures
References
-
- Garnock-Jones KP, Scott LJ. Fospropofol. Drugs. 2010;70(4):469–77. - PubMed
-
- Mueller SW, Moore GD, MacLaren R. Fospropofol Disodium for Procedural Sedation: Emerging Evidence of its Value? Clinical Medicine Insights: Therapeutics. 2010;2:513–22.
-
- Welliver M, Rugari SM. New drug, fospropofol disodium: a propofol prodrug. AANA J. 2009;77(4):301–8. - PubMed
-
- Fospropofol Disodium Injection NDA 22-244,MGI PHARMA, Inc. Briefing materials for the anaesthetic and life support drugs advisory committee to the division of anaesthesia, analgesia, and rheumatology products (DAARP)/CDER/FDA. 2008. May 7, [Accessed 2010 Aug 17]. Available from: http: www.fda.gov/ ohrms/dockets/ ac/08 /briefing/2008-4354b1-02-MGI.pdf.
LinkOut - more resources
Full Text Sources
Other Literature Sources