Rhodium-Catalyzed C-H Amination - An Enabling Method for Chemical Synthesis
- PMID: 21804756
- PMCID: PMC3143484
- DOI: 10.1021/op200046v
Rhodium-Catalyzed C-H Amination - An Enabling Method for Chemical Synthesis
Abstract
Reaction methods for selective C-H amination are finding ever-increasing utility for the preparation of nitrogen-derived fine chemicals. This brief account highlights the remarkable versatility of dirhodium-based catalysts for promoting oxidation of aliphatic C-H centers in both intra- and intermolecular reaction processes.
Figures
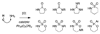
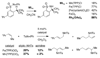
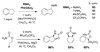
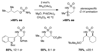

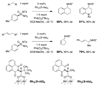



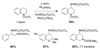
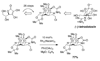

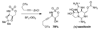
References
-
- Yu J-Q, Shi Z, editors. “C–H Activation”, Topics in Current Chemistry. Berlin: Springer-Verlag; 2010. pp. 1–384.
- Dyker G, editor. “Handbook of C–H Transformations: Applications in Organic Synthesis”. Weinheim: Wiley-VCH; 2005. pp. 1–688.
-
- Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. - PubMed
- Crabtree RH. Chem. Rev. 2010;110:575. and references therein. - PubMed
- Giri R, Shi B-F, Engle KM, Maugel N, Yu J-Q. Chem. Soc. Rev. 2009;38:3242. - PubMed
- Hartwig JF. Nature. 2008;455:314. - PMC - PubMed
- Murahashi S-I, Zhang D. Chem. Soc. Rev. 2008;37:1490. - PubMed
- Dick AR, Sanford MS. Tetrahedron. 2006;62:2439.
- Godula K, Sammes D. Science. 2006;312:67. - PubMed
- Labinger JA, Bercaw JE. Nature. 2002;417:507. - PubMed
-
- Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. New York: John Wiley & Sons; 1995.
-
-
Du Bois J. CHEMTRACTS – Org. Chem. 2005;18:1. Also, see: McNeill E, Du Bois J. J. Am. Chem. Soc. 2010;132:10202. Litvinas ND, Brodsky BH, Du Bois J. Angew. Chem. Int. Ed. 2009;48:4513. Brodsky BH, Du Bois J. J. Am. Chem. Soc. 2005;127:15391.
-
-
-
For some leading references, see: Liu Y, Wei J, Che C-M. Chem. Commun. 2010;46:6926. Lu H-J, Subbarayan V, Tai J-R, Zhang PX. Organometallics. 2010;29:389. Badiei YM, Dinescu A, Dai X, Palomino RM, Heinemann FW, Cundari TR, Warren TH. Angew. Chem. Int. Ed. 2008;47:9961. Shen M, Leslie BE, Driver TG. Angew. Chem. Int. Ed. 2008;47:5056. Li Z, Capretto DA, Rahaman R, He C. Angew. Chem. Int. Ed. 2007;46:5184. Lebel H, Huard K, Lectard S. Org. Lett. 2007;9:639. Liang C, Robert-Pedlard F, Fruit C, Müller P, Dodd RH, Dauban P. Angew. Chem. Int. Ed. 2006;45:4641.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
