Metathesis access to monocyclic iminocyclitol-based therapeutic agents
- PMID: 21804866
- PMCID: PMC3135129
- DOI: 10.3762/bjoc.7.81
Metathesis access to monocyclic iminocyclitol-based therapeutic agents
Abstract
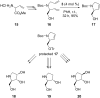
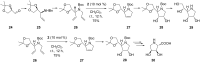
By focusing on recent developments on natural and non-natural azasugars (iminocyclitols), this review bolsters the case for the role of olefin metathesis reactions (RCM, CM) as key transformations in the multistep syntheses of pyrrolidine-, piperidine- and azepane-based iminocyclitols, as important therapeutic agents against a range of common diseases and as tools for studying metabolic disorders. Considerable improvements brought about by introduction of one or more metathesis steps are outlined, with emphasis on the exquisite steric control and atom-economical outcome of the overall process. The comparative performance of several established metathesis catalysts is also highlighted.
Keywords: Ru-alkylidene catalysts; azasugars; iminocyclitols; natural products; olefin metathesis.
Figures

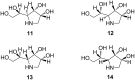


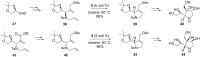
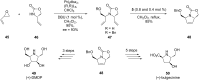
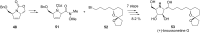


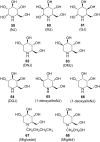
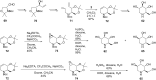



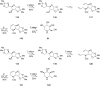




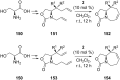
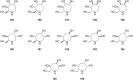
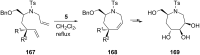

References
-
- Compain P, Martin O R, editors. Iminosugars: From Synthesis to Therapeutic Applications. Weinheim, Germany: Wiley-VCH; 2007.
-
- Stütz A E. Iminosugars as Glycosidase Inhibitors: Nojirimycin and Beyond. Weinheim, Germany: Wiley-VCH; 1999.
-
- Martin O R, Compain P, editors. Iminosugars: Recent Insights into Their Bioactivity Potential as Therapeutic Agents. Curr Top Med Chem. 2003;3:471–591.
LinkOut - more resources
Full Text Sources
Other Literature Sources
