Gold-catalyzed propargylic substitutions: Scope and synthetic developments
- PMID: 21804883
- PMCID: PMC3135077
- DOI: 10.3762/bjoc.7.99
Gold-catalyzed propargylic substitutions: Scope and synthetic developments
Abstract
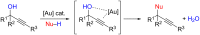
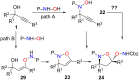
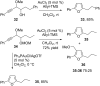
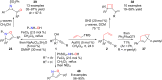
This personal account summarizes our recent developments in gold-catalyzed direct substitutions on propargylic (allylic, benzylic) alcohols, with various nucleophiles (and bi-nucleophiles) based on the σ- and/or π-acidity of gold(III) complexes. Synthetic developments are also briefly described.
Keywords: direct substitutions; gold; isoxazolines; propargylic substitutions.
Figures
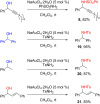
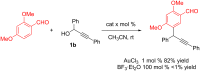
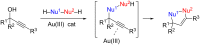
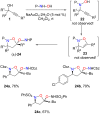
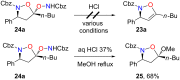
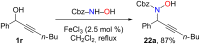
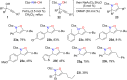
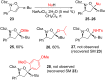
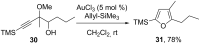
References
-
- Constable D J C, Dunn P J, Hayler J D, Humphrey G R, Leazer J L, Linderman R J, Lorenz K, Manley J, Pearlman B A, Wells A, et al. Green Chem. 2007;9:411–420. doi: 10.1039/b703488c. - DOI
-
- Emer E, Sinisi R, Capdevila M G, Petruzziello D, De Vincentiis F, Cozzi P G. Eur J Org Chem. 2011:647–666. doi: 10.1002/ejoc.201001474. - DOI
-
- Bisaro F, Prestat G, Vitale M, Poli G. Synlett. 2002:1823–1826. doi: 10.1055/s-2002-34884. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
