Recent advances in the gold-catalyzed additions to C-C multiple bonds
- PMID: 21804887
- PMCID: PMC3135236
- DOI: 10.3762/bjoc.7.103
Recent advances in the gold-catalyzed additions to C-C multiple bonds
Abstract
C-O, C-N and C-C bonds are the most widespread types of bonds in nature, and are the cornerstone of most organic compounds, ranging from pharmaceuticals and agrochemicals to advanced materials and polymers. Cationic gold acts as a soft and carbophilic Lewis acid and is considered one of the most powerful activators of C-C multiple bonds. Consequently, gold-catalysis plays an important role in the development of new strategies to form these bonds in more convenient ways. In this review, we highlight recent advances in the gold-catalyzed chemistry of addition of X-H (X = O, N, C) bonds to C-C multiple bonds, tandem reactions, and asymmetric additions. This review covers gold-catalyzed organic reactions published from 2008 to the present.
Keywords: C−C multiple bonds; asymmetric addition; catalysis; gold; tandem reaction.
Figures

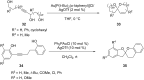
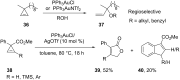
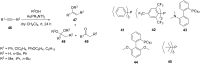
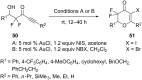

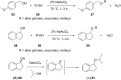
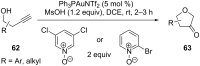
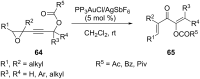
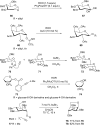

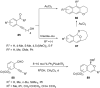
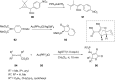
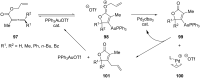
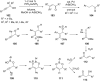

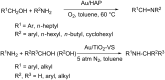
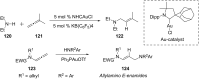

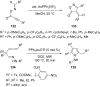

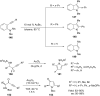
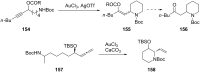
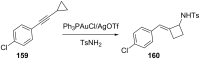
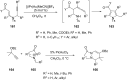
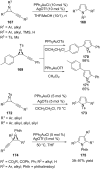
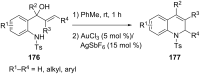
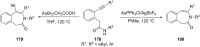

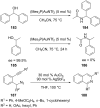
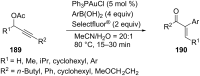

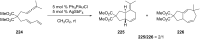
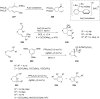
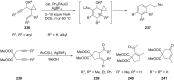
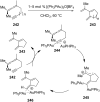
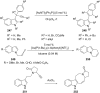
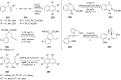
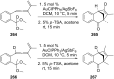
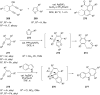
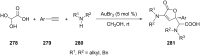
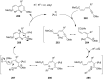
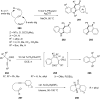
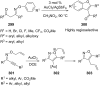
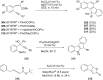
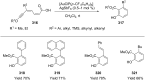
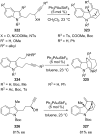
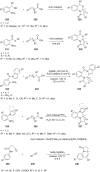
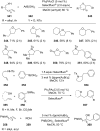
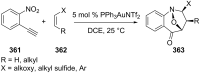
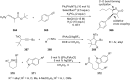
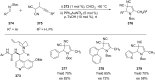
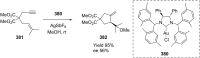
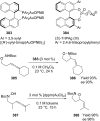
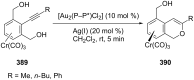
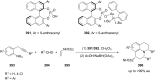
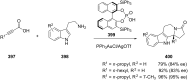
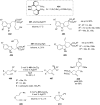
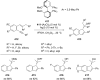
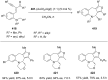
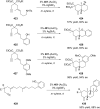
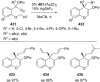
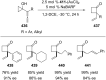
References
-
- Hashmi A S K, Bührle M. Aldrichimica Acta. 2010;43:27–33.
-
- Patil N T, Yamamoto Y. ARKIVOC. 2007;(v):6–19.
-
- Hashmi A S K. Pure Appl Chem. 2010;82:657–668. doi: 10.1351/Pac-Con-09-10-17. - DOI
LinkOut - more resources
Full Text Sources
