Sphingosine 1-phosphate in coagulation and inflammation
- PMID: 21805322
- PMCID: PMC3237867
- DOI: 10.1007/s00281-011-0287-3
Sphingosine 1-phosphate in coagulation and inflammation
Abstract
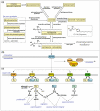
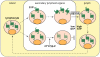
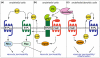
Sphingosine 1-phosphate (S1P) is a lipid mediator produced from sphingomyelin by the sequential enzymatic actions of sphingomyelinase, ceramidase, and sphingosine kinase. Five subtypes of cell surface G-protein-coupled receptors, S1P(1-5), mediate the actions of S1P in various organs systems, most notably cardiovascular, immune, and central nervous systems. S1P is enriched in blood and lymph but is present at much lower concentrations in interstitial fluids of tissues. This vascular S1P gradient is important for the regulation of trafficking of various immune cells. FTY720, which was recently approved for the treatment of relapsing-remitting multiple sclerosis, potently sequesters lymphocytes into lymph nodes by functionally antagonizing the activity of the S1P(1) receptor. S1P also plays critical roles in the vascular barrier integrity, thereby regulating inflammation, tumor metastasis, angiogenesis, and atherosclerosis. Recent studies have also revealed the involvement of S1P signaling in coagulation and in tumor necrosis factor α-mediated signaling. This review highlights the importance of S1P signaling in these inflammatory processes as well as the contribution of each receptor subtype, which exhibits both cooperative and redundant functions.
Figures
References
-
- Westerlund B, Slotte JP. How the molecular features of glycosphingolipids affect domain formation in fluid membranes. Biochim Biophys Acta. 2009;1788:194–201. - PubMed
-
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. - PubMed
-
- Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

