Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin
- PMID: 21806041
- PMCID: PMC3321232
- DOI: 10.1021/tx2002433
Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin
Abstract
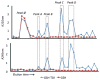
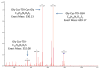
Glutathione has previously been identified as a reaction target for toluene diisocyanate (TDI) in vitro and in vivo, and has been suggested to contribute to toxic and allergic reactions to exposure. In this study, the reactivity of reduced glutathione (GSH) with TDI in vitro was further investigated using a mixed phase (vapor/liquid) exposure system to model the in vivo biophysics of exposure in the lower respiratory tract. HPLC/MS/MS was used to characterize the observed reaction products. Under the conditions tested, the major reaction products between TDI vapor and GSH were S-linked bis(GSH)-TDI and to a lesser extent mono(GSH)-TDI conjugates (with one N═C═O hydrolyzed). The vapor-phase-generated GSH-TDI conjugates were capable of transcarbamoylating human albumin in a pH-dependent manner, resulting in changes in the self-protein's conformation/charge, on the basis of electrophoretic mobility under native conditions. Specific sites of human albumin-TDI conjugation, mediated by GSH-TDI, were identified (Lys(73), Lys(159), Lys(190), Lys(199), Lys(212), Lys(351), Lys(136/137), Lys(413/414), and Lys(524/525)) along with overlap with those susceptible to direct conjugation by TDI. Together, the data extend the proof-of-principle for GSH to act as a "shuttle" for a reactive form of TDI, which could contribute to clinical responses to exposure.
Figures






References
-
- Baur X, Marek W, Ammon J, Czuppon AB, Marczynski B, Raulf-Heimsoth M, Roemmelt H, Fruhmann G. Respiratory and other hazards of isocyanates. Int Arch Occup Environ Health. 1994;66:141–152. - PubMed
-
- Collins MA. Toxicology of toluene diisocyanate. Appl Occup Environ Hyg. 2002;17:846–855. - PubMed
-
- Lindberg HK, Korpi A, Santonen T, Sakkinen K, Jarvela M, Tornaeus J, Ahonen N, Jarventaus H, Pasanen AL, Rosenberg C, Norppa H. Micronuclei, hemoglobin adducts and respiratory tract irritation in mice after inhalation of toluene diisocyanate (TDI) and 4,4′-methylenediphenyl diisocyanate (MDI) Mutat Res 2011 - PubMed
-
- NIOSH. DHHS (NIOSH) Publication Number 96–111. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health; 1996. Preventing Asthma and Death from Diisocyanate Exposure.
-
- NIOSH. DHHS (NIOSH) Publication No 90–101. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health; 1989. Current Intelligence Bulletin 53: toluene diisocyanate (TDI) and toluenediamine (TDA); evidence of carcinogenicity.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

