Label-free oxygen-metabolic photoacoustic microscopy in vivo
- PMID: 21806264
- PMCID: PMC3144973
- DOI: 10.1117/1.3594786
Label-free oxygen-metabolic photoacoustic microscopy in vivo
Abstract
Almost all diseases, especially cancer and diabetes, manifest abnormal oxygen metabolism. Accurately measuring the metabolic rate of oxygen (MRO(2)) can be helpful for fundamental pathophysiological studies, and even early diagnosis and treatment of disease. Current techniques either lack high resolution or rely on exogenous contrast. Here, we propose label-free metabolic photoacoustic microscopy (mPAM) with small vessel resolution to noninvasively quantify MRO(2) in vivo in absolute units. mPAM is the unique modality for simultaneously imaging all five anatomical, chemical, and fluid-dynamic parameters required for such quantification: tissue volume, vessel cross-section, concentration of hemoglobin, oxygen saturation of hemoglobin, and blood flow speed. Hyperthermia, cryotherapy, melanoma, and glioblastoma were longitudinally imaged in vivo. Counterintuitively, increased MRO(2) does not necessarily cause hypoxia or increase oxygen extraction. In fact, early-stage cancer was found to be hyperoxic despite hypermetabolism.
Figures

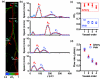
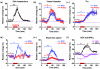
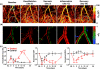
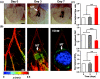

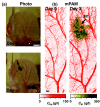
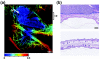
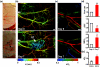
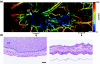
References
-
- Vaupel P., Kallinowski F., and Okunieff P., “Blood-flow, oxygen and nutrient supply, and metabolic microenvironment of human-tumors – a review,” Cancer Res. 49(23), 6449–6465 (1989). - PubMed
-
- Cheng K., Ho K., Stokes R., Scott C., Lau S. M., Hawthorne W. J., O’Connell P. J., Loudovaris T., Kay T. W., Kulkarni R. N., Okada T., Wang X. H. L., Yim S. H., Shah Y., Grey S. T., Biankin A. V., Kench J. G., Laybutt D. R., Gonzalez F. J., Kahn C. R., and Gunton J. E., “Hypoxia-inducible factor-1 alpha regulates beta cell function in mouse and human islets,” J. Clin. Invest. 120(6), 2171–2183 (2010).10.1172/JCI35846 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

