Thermodynamics-based drug design: strategies for inhibiting protein-protein interactions
- PMID: 21806377
- PMCID: PMC3164996
- DOI: 10.4155/fmc.11.81
Thermodynamics-based drug design: strategies for inhibiting protein-protein interactions
Abstract
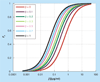
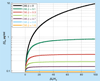
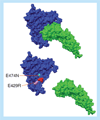
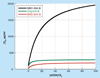
The inhibition of protein-protein interactions and their ensuing signaling processes play an increasingly important role in modern medicine. Small molecular-weight inhibitors that can be administered orally are the preferred approach but efficient strategies for developing them are not yet generally available. Due to the large size difference between the protein-protein interface and the small molecule, inhibitor interactions are expected to extend to only a small region of the interface. If this is the case, classical competitive inhibition may be hard to achieve. In addition, competitive inhibition wastes binding energy that can be effectively used to inhibit signaling. The best and most energy-efficient approach would be the development of small molecules that bind at the protein-protein interface and inhibit the signaling process without displacing the protein ligand. This approach seems feasible knowing that the binding energy is not evenly distributed within the binding interface but concentrated in discrete hotspots, and that the initiation of signaling may not overlap with those hotspots. We outline a general protein-protein inhibition model that extends from competitive to noncompetitive scenarios and apply it to the development of HIV-1 gp120-CD4 inhibitors. This rigorous model can be easily applied to the analysis of protein-protein inhibition data and used as a tool in the optimization of inhibitor molecules.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures


Similar articles
-
Structure-based design, synthesis and validation of CD4-mimetic small molecule inhibitors of HIV-1 entry: conversion of a viral entry agonist to an antagonist.Acc Chem Res. 2014 Apr 15;47(4):1228-37. doi: 10.1021/ar4002735. Epub 2014 Feb 6. Acc Chem Res. 2014. PMID: 24502450 Free PMC article. Review.
-
Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120.Biochemistry. 2006 Sep 12;45(36):10973-80. doi: 10.1021/bi061193r. Biochemistry. 2006. PMID: 16953583 Free PMC article.
-
Optimization of CD4/gp120 inhibitors by thermodynamic-guided alanine-scanning mutagenesis.Chem Biol Drug Des. 2013 Jan;81(1):72-8. doi: 10.1111/cbdd.12075. Chem Biol Drug Des. 2013. PMID: 23066870 Free PMC article.
-
Conformational and structural features of HIV-1 gp120 underlying the dual receptor antagonism by cross-reactive neutralizing antibody m18.Biochemistry. 2011 Apr 12;50(14):2756-68. doi: 10.1021/bi101160r. Epub 2011 Mar 18. Biochemistry. 2011. PMID: 21351734 Free PMC article.
-
Between a rock and a hard place?Nat Chem Biol. 2006 Mar;2(3):112-8. doi: 10.1038/nchembio0306-112. Nat Chem Biol. 2006. PMID: 16484997 Review.
Cited by
-
Isothermal titration calorimetry (ITC): a standard operating procedure (SOP).Eur Biophys J. 2021 May;50(3-4):363-371. doi: 10.1007/s00249-021-01509-5. Epub 2021 Mar 4. Eur Biophys J. 2021. PMID: 33665758
-
An in Silico Approach Reveals the Potential Function of Cyanidin-3-o-glucoside of Red Rice in Inhibiting the Advanced Glycation End Products (AGES)-Receptor (RAGE) Signaling Pathway.Acta Inform Med. 2020 Sep;28(3):170-179. doi: 10.5455/aim.2020.28.170-179. Acta Inform Med. 2020. PMID: 33417643 Free PMC article.
-
Sekikaic acid and lobaric acid target a dynamic interface of the coactivator CBP/p300.Angew Chem Int Ed Engl. 2012 Nov 5;51(45):11258-62. doi: 10.1002/anie.201206815. Epub 2012 Oct 8. Angew Chem Int Ed Engl. 2012. PMID: 23042634 Free PMC article.
-
Engineering a genetically encoded competitive inhibitor of the KEAP1-NRF2 interaction via structure-based design and phage display.Protein Eng Des Sel. 2016 Jan;29(1):1-9. doi: 10.1093/protein/gzv055. Epub 2015 Oct 20. Protein Eng Des Sel. 2016. PMID: 26489878 Free PMC article.
-
A miniaturized mode-of-action profiling platform enables high throughput characterization of the molecular and cellular dynamics of EZH2 inhibition.Sci Rep. 2024 Jan 19;14(1):1739. doi: 10.1038/s41598-023-50964-x. Sci Rep. 2024. PMID: 38242973 Free PMC article.
References
-
- Wells JA, Mcclendon CL. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature. 2007;450(7172):1001–1009. - PubMed
-
- Zinzalla G, Thurston DE. Targeting protein–protein interactions for therapeutic intervention: a challenge for the future. Future Med. Chem. 2009;1(1):65–93. - PubMed
-
- Callen JP. Complications and adverse reactions in the use of newer biologic agents. Semin. Cutan. Med. Surg. 2007;26(1):6–14. - PubMed
-
- Banner DW, D’arcy A, Janes W, et al. Crystal structure of the soluble human 55 kd TNF receptor-human TNF beta complex: implications for TNF receptor activation. Cell. 1993;73(3):431–445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
