Comparative analyses imply that the enigmatic Sigma factor 54 is a central controller of the bacterial exterior
- PMID: 21806785
- PMCID: PMC3162934
- DOI: 10.1186/1471-2164-12-385
Comparative analyses imply that the enigmatic Sigma factor 54 is a central controller of the bacterial exterior
Abstract
Background: Sigma-54 is a central regulator in many pathogenic bacteria and has been linked to a multitude of cellular processes like nitrogen assimilation and important functional traits such as motility, virulence, and biofilm formation. Until now it has remained obscure whether these phenomena and the control by Sigma-54 share an underlying theme.
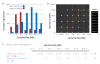
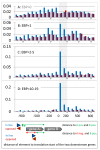
Results: We have uncovered the commonality by performing a range of comparative genome analyses. A) The presence of Sigma-54 and its associated activators was determined for all sequenced prokaryotes. We observed a phylum-dependent distribution that is suggestive of an evolutionary relationship between Sigma-54 and lipopolysaccharide and flagellar biosynthesis. B) All Sigma-54 activators were identified and annotated. The relation with phosphotransfer-mediated signaling (TCS and PTS) and the transport and assimilation of carboxylates and nitrogen containing metabolites was substantiated. C) The function annotations, that were represented within the genomic context of all genes encoding Sigma-54, its activators and its promoters, were analyzed for intra-phylum representation and inter-phylum conservation. Promoters were localized using a straightforward scoring strategy that was formulated to identify similar motifs. We found clear highly-represented and conserved genetic associations with genes that concern the transport and biosynthesis of the metabolic intermediates of exopolysaccharides, flagella, lipids, lipopolysaccharides, lipoproteins and peptidoglycan.
Conclusion: Our analyses directly implicate Sigma-54 as a central player in the control over the processes that involve the physical interaction of an organism with its environment like in the colonization of a host (virulence) or the formation of biofilm.
Figures




References
-
- Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466. - PubMed
-
- Kolter R, Siegele DA, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. - PubMed
-
- Hengge R. Proteolysis of sigmaS (RpoS) and the general stress response in Escherichia coli. Res Microbiol. 2009;160(9):667–676. - PubMed
-
- Zhao K, Liu M, Burgess RR. The global transcriptional response of Escherichia coli to induced sigma 32 protein involves sigma 32 regulon activation followed by inactivation and degradation of sigma 32 in vivo. J Biol Chem. 2005;280(18):17758–17768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

