Large-scale RNAi screen of G protein-coupled receptors involved in larval growth, molting and metamorphosis in the red flour beetle
- PMID: 21806814
- PMCID: PMC3163568
- DOI: 10.1186/1471-2164-12-388
Large-scale RNAi screen of G protein-coupled receptors involved in larval growth, molting and metamorphosis in the red flour beetle
Abstract
Background: The G protein-coupled receptors (GPCRs) belong to the largest superfamily of integral cell membrane proteins and play crucial roles in physiological processes including behavior, development and reproduction. Because of their broad and diverse roles in cellular signaling, GPCRs are the therapeutic targets for many prescription drugs. However, there is no commercial pesticide targeting insect GPCRs. In this study, we employed functional genomics methods and used the red flour beetle, Tribolium castaneum, as a model system to study the physiological roles of GPCRs during the larval growth, molting and metamorphosis.
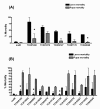
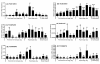
Results: A total of 111 non-sensory GPCRs were identified in the T. castaneum genome. Thirty-nine of them were not reported previously. Large-scale RNA interference (RNAi) screen was used to study the function of all these GPCRs during immature stages. Double-stranded RNA (dsRNA)-mediated knockdown in the expression of genes coding for eight GPCRs caused severe developmental arrest and ecdysis failure (with more than 90% mortality after dsRNA injection). These GPCRs include dopamine-2 like receptor (TC007490/D2R) and latrophilin receptor (TC001872/Cirl). The majority of larvae injected with TC007490/D2R dsRNA died during larval stage prior to entering pupal stage, suggesting that this GPCR is essential for larval growth and development.
Conclusions: The results from our study revealed the physiological roles of some GPCRs in T. castaneum. These findings could help in development of novel pesticides targeting these GPCRs.
Figures

Similar articles
-
Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum.Mech Dev. 2008 Mar-Apr;125(3-4):299-313. doi: 10.1016/j.mod.2007.11.001. Epub 2007 Nov 17. Mech Dev. 2008. PMID: 18083350 Free PMC article.
-
A Major Facilitator Superfamily protein encoded by TcMucK gene is not required for cuticle pigmentation, growth and development in Tribolium castaneum.Insect Biochem Mol Biol. 2014 Jun;49:43-8. doi: 10.1016/j.ibmb.2014.03.007. Epub 2014 Mar 28. Insect Biochem Mol Biol. 2014. PMID: 24681434
-
FoxO mediates the timing of pupation through regulating ecdysteroid biosynthesis in the red flour beetle, Tribolium castaneum.Gen Comp Endocrinol. 2018 Mar 1;258:149-156. doi: 10.1016/j.ygcen.2017.05.012. Epub 2017 May 16. Gen Comp Endocrinol. 2018. PMID: 28526479
-
A genome-wide inventory of neurohormone GPCRs in the red flour beetle Tribolium castaneum.Front Neuroendocrinol. 2008 Jan;29(1):142-65. doi: 10.1016/j.yfrne.2007.10.003. Epub 2007 Oct 24. Front Neuroendocrinol. 2008. PMID: 18054377 Review.
-
G protein coupled receptors as targets for next generation pesticides.Insect Biochem Mol Biol. 2015 Dec;67:27-37. doi: 10.1016/j.ibmb.2015.07.014. Epub 2015 Jul 29. Insect Biochem Mol Biol. 2015. PMID: 26226649 Review.
Cited by
-
Identification of G protein-coupled receptors required for vitellogenin uptake into the oocytes of the red flour beetle, Tribolium castaneum.Sci Rep. 2016 Jun 9;6:27648. doi: 10.1038/srep27648. Sci Rep. 2016. PMID: 27277501 Free PMC article.
-
Unveiling the Role of Two Rhodopsin-like GPCR Genes in Insecticide-Resistant House Flies, Musca domestica.Int J Mol Sci. 2024 Oct 2;25(19):10618. doi: 10.3390/ijms251910618. Int J Mol Sci. 2024. PMID: 39408947 Free PMC article.
-
Importance of Taiman in Larval-Pupal Transition in Leptinotarsa decemlineata.Front Physiol. 2019 Jun 13;10:724. doi: 10.3389/fphys.2019.00724. eCollection 2019. Front Physiol. 2019. PMID: 31263425 Free PMC article.
-
Development and Interrogation of a Transcriptomic Resource for the Giant Triton Snail (Charonia tritonis).Mar Biotechnol (NY). 2021 Jun;23(3):501-515. doi: 10.1007/s10126-021-10042-7. Epub 2021 Jun 30. Mar Biotechnol (NY). 2021. PMID: 34191212 Free PMC article.
-
Evolutionary conserved peptide and glycoprotein hormone-like neuroendocrine systems in C. elegans.Mol Cell Endocrinol. 2024 Apr 15;584:112162. doi: 10.1016/j.mce.2024.112162. Epub 2024 Jan 28. Mol Cell Endocrinol. 2024. PMID: 38290646 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

