Amnion formation in the mouse embryo: the single amniochorionic fold model
- PMID: 21806820
- PMCID: PMC3163621
- DOI: 10.1186/1471-213X-11-48
Amnion formation in the mouse embryo: the single amniochorionic fold model
Abstract
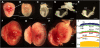
Background: Despite the detailed knowledge obtained over the last decade on the molecular regulation of gastrulation in amniotes, the process of amnion development has been poorly described and illustrated in mice, and conflicting descriptions exist. Understanding the morphogenesis and development not only of the early mouse embryo, but also of its extraembryonic tissues, is crucial for correctly interpreting fate-mapping data and mouse mutants with gastrulation defects. Moreover, the recent isolation from amnion of cells with stem cell features further argues for a better understanding of the process of amnion formation. Here, we revisit the highly dynamic process of amnion formation in the mouse. Amnion development starts early during gastrulation and is intimately related to the formation of the exocoelom and the expansion of the amniotic fold. The authoritative description involves the fusion of two amniotic folds, a big posterior and a smaller anterior fold. We challenged this 'two amniotic folds' model by performing detailed histomorphological analyses of dissected, staged embryos and 3D reconstructions using historical sections.
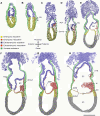
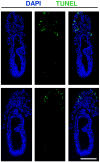
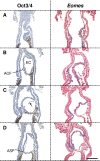
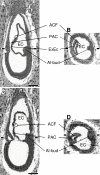
Results: A posterior fold of extraembryonic ectoderm and associated epiblast is formed early during gastrulation by accumulation of extraembryonic mesoderm posterior to the primitive streak. Previously called the "posterior amniotic fold", we rename it the "amniochorionic fold" (ACF) because it forms both amnion and chorion. Exocoelom formation within the ACF seems not to involve apoptosis within the mesoderm. The ACF and exocoelom expand without disrupting the anterior junction of epiblast, extraembryonic ectoderm and visceral endoderm. No separate anterior fold is formed; its absence was confirmed in 3D reconstructions. Amnion and chorion closure is eccentric, close to the anterior margin of the egg cylinder: we name it the "anterior separation point".
Conclusions: Here, we reconcile previous descriptions of amnion formation and provide new nomenclature, as well as an animation, that clarify and emphasize the arrangement of the tissues that contribute to amnion development and the dynamics of the process. According to our data, the amnion and the chorion are formed by a single amniochorionic fold initiated posteriorly. Finally, we give an overview on mutant mouse models with impaired amnion development.
Figures

References
-
- Schmidt W. The amniotic fluid compartment: the fetal habitat. Adv Anat Embryol Cell Biol. 1992;127:1–100. - PubMed
-
- Gardner RL. The relationship between cell lineage and differentiation in the early mouse embryo. Results Probl Cell Differ. 1978;9:205–41. - PubMed
-
- Kaufman MH. The Atlas of Mouse Development. London: Academic Press; 1992.
-
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

