Spatiotemporal expression of SERPINE2 in the human placenta and its role in extravillous trophoblast migration and invasion
- PMID: 21806836
- PMCID: PMC3161939
- DOI: 10.1186/1477-7827-9-106
Spatiotemporal expression of SERPINE2 in the human placenta and its role in extravillous trophoblast migration and invasion
Abstract
Background: SERPINE2, one of the potent serpins belonging to the plasminogen activator (PA) system, is involved in the tissue remodeling. We previously demonstrated the expression patterns of Serpine2 in the mouse placenta and uterus, indicating that Serpine2 is a major PA inhibitor in the placenta and uterus during the estrous cycle, pregnancy, and lactation. In this study, we further investigated the expression pattern of SERPINE2 in the human placenta and explored possible functional roles of SERPINE2 in regulating trophoblast activity.
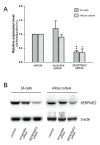
Methods: Placental tissues from various trimesters were collected for real-time reverse-transcription polymerase chain reaction quantification. Immunohistochemical staining was performed in placental tissues to assure localization of SERPINE2. SERPINE2 small interfering (si) RNA was applied to suppress its expression in villous explants and extravillous trophoblast-like 3A cells. Subsequent experiments to evaluate SERPINE2 levels, villous outgrowth, trophoblast invasion, and tube formation were performed.
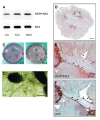
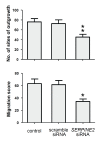
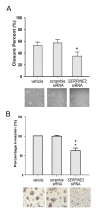
Results: SERPINE2 messenger RNA was detected in the human placenta during pregnancy with the highest levels in the third trimester. The SERPINE2 protein was present in villous syncytiotrophoblasts and trophoblasts of chorionic villi for anti-SERPINE2 immunostaining. Extravillous trophoblasts in the chorionic plate and basal plate confronting the invasive face of anchoring villi were also positive. In most decidual cells, SERPINE2 was observed in the cytoplasm. In addition, fibrinoid deposit was weakly immunoreactive. Introduction of SERPINE2 siRNA into villous explants and trophoblast cells led to significantly reduced villous outgrowth, and trophoblastic migration and invasion. Moreover, capillary-like network formation of 3A cells in Matrigel was greatly attenuated by SERPINE2 siRNA and SERPINE2 antiserum.
Conclusions: These data identify the temporal and spatial SERPINE2 distribution in the human placenta and suggest its possible role in modulating tissue remodeling of extravillous trophoblasts in the placenta during pregnancy.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

