Comparison of immune response generated against Japanese encephalitis virus envelope protein expressed by DNA vaccines under macrophage associated versus ubiquitous expression promoters
- PMID: 21806845
- PMCID: PMC3161000
- DOI: 10.1186/1743-422X-8-382
Comparison of immune response generated against Japanese encephalitis virus envelope protein expressed by DNA vaccines under macrophage associated versus ubiquitous expression promoters
Abstract
Background: Japanese encephalitis virus (JEV) is the leading cause of viral encephalitis, with ~50,000 cases reported annually worldwide. Vaccination is the only measure for prevention. Recombinant vaccines are an efficient and safe alternative for formalin inactivated or live attenuated vaccines. Nowadays, incorporation of molecular adjuvants has been the main strategy for melioration of vaccines. Our attempt of immunomodulation is based on targeting antigen presenting cells (APC) "majorly macrophages" by using macrosialin promoter. We have compared the immune response of the constructed plasmids expressing JEV envelope (E) protein under the control of aforesaid promoter and cytomegalovirus (CMV) immediate early promoter in mouse model. Protection of immunized mice from lethal challenge with JEV was also studied.
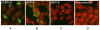
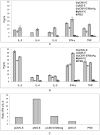
Results: The E protein was successfully expressed in the macrophage cell line and was detected using immunofluorescence assay (IFA) and Western blotting. APC expressing promoter showed comparable expression to CMV promoter. Immunization of mice with either of the plasmids exhibited induction of variable JEV neutralizing antibody titres and provided protection from challenge with a lethal dose of JEV. Immune splenocytes showed proliferative response after stimulation with the JEV antigen (Ag), however, it was higher for CMV promoter. The magnitude of immunity provided by APC dominant promoter was non-significantly lower in comparison to CMV promoter. More importantly, immune response directed by APC promoter was skewed towards Th1 type in comparison to CMV promoter, this was evaluated by cytokine secretion profile of immune splenocytes stimulated with JEV Ag.
Conclusions: Thus, our APC-expressing DNA vaccination approach induces comparable immunity in comparison to ubiquitous promoter construct. The predominant Th1 type immune responses provide opportunities to further test its potency suitable for response in antiviral or anticancer vaccines.
Figures


References
-
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(12 Suppl):S98–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

