Neurotensin signaling activates microRNAs-21 and -155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors
- PMID: 21806946
- PMCID: PMC4442678
- DOI: 10.1053/j.gastro.2011.07.038
Neurotensin signaling activates microRNAs-21 and -155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors
Abstract
Background & aims: Neurotensin promotes inflammation and colon cancer via the neurotensin-1 receptor (NTR1). MicroRNAs (miR) regulate protein synthesis by degrading or preventing translation of mRNAs. We analyzed expression of 365 different microRNAs by human colonic epithelial cells (NCM460) after activation of NTR1.
Methods: We performed microarray analysis of mRNA expression by neurotensin-stimulated NCM460 cells that overexpressed NTR1. Nuclear factor-κB (NF-κB) binding sites were identified and tumorigenesis was assessed using soft agar assays and xenograft analysis of severe combined immunodeficiency mice. Targets of neurotensin-regulated microRNAs were identified via bioinformatic, real-time polymerase chain reaction, and immunoblot analyses. We analyzed RNA samples from human normal colon and tumor samples.
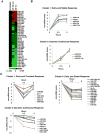
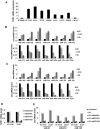
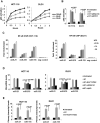
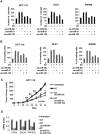
Results: Neurotensin stimulated differential expression of 38 microRNAs, including miR-21 and miR-155, which have been associated with tumor growth and contain NF-κB binding sites. Neurotensin expression increased colony formation by HCT-116 cells. Blocking miR-21 and/or miR-155 prevented colony formation (P < .001). In mice, intraperitoneal administration of neurotensin increased the growth rate of HCT-116 xenograft tumors; blocking miR-21 and/or miR-155 slowed this tumor growth. Neurotensin activated Akt in HCT-116 cells; this effect was inhibited by blocking miR-21 and/or miR-155 (P < .001). Neurotensin activated AKT through miR-155-mediated suppression of the phosphatase protein phosphatase 2A catalytic subunit alpha (PPP2CA). Levels of phosphatase and tensin homolog (PTEN) and suppressor of cytokine signaling 1 (SOCS1) mRNA, potential targets of miR-21 and miR-155, respectively, were down-regulated by these miRs. Levels of NTR1, miR-21, and miR-155 increased significantly in human colon tumor samples, compared with normal tissues, whereas PPP2CA, SOCS1, and PTEN mRNAs were reduced significantly.
Conclusions: NTR1 activation stimulates expression of miR-21 and miR-155 in colonocytes, via Akt and NF-κB, to down-regulate PTEN and SOCS1 and promote growth of tumors in mice. Levels of NTR1, miR-21, and miR-155 increase in human colon tumor samples and correlate with tumor stage.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8. - PubMed
-
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. - PubMed
-
- Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull. 2006;29:903–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

