Deferiprone modulates in vitro responses by peripheral blood T cells from control and relapsing-remitting multiple sclerosis subjects
- PMID: 21807124
- PMCID: PMC3204173
- DOI: 10.1016/j.intimp.2011.07.007
Deferiprone modulates in vitro responses by peripheral blood T cells from control and relapsing-remitting multiple sclerosis subjects
Abstract
T cells are important mediators of autoimmune inflammation in relapsing-remitting multiple sclerosis (RRMS). Previous studies found that deferiprone, an iron chelator, suppressed disease activity in a mouse model of multiple sclerosis, and inhibition of T cell proliferation was implicated as a putative mechanism. The objective of the present study was to examine the effects of deferiprone on suppressing in vitro responses of T cells from control and RRMS subjects. Peripheral blood T cells were co-stimulated with anti-CD3+anti-CD28 and cultured with or without interleukin 2 (IL-2). Proliferating CD4+ T cells from control and RRMS subjects, cultured with or without IL-2, decreased in response to 75 μM deferiprone, although the extent of decreased proliferation of CD4+ T cells from RRMS subjects was less than for control subjects. Proliferating CD8+ T cells from control subjects, cultured with or without IL-2, also decreased in response to 75 μM deferiprone, and this decrease was seen in proliferating CD8+ T cells from RRMS cultured with IL-2. CD4+CD25+ and CD8+CD25+ cells from control subjects, cultured with or without IL-2, declined in 75 μM deferiprone, but the decrease was smaller than for the CD4+ and CD8+ proliferative responses. CD4+CD25+ and CD8+CD25+ cells from RRMS subjects showed more variability than for control subjects, but CD4+CD25+ cultured with IL-2 and CD8+CD25+ cells cultured without IL-2 significantly declined in 75 μM deferiprone. CD4+FoxP3+ and CD4+CD25+FoxP3+ cells tended to remain constant or increase. In summary, deferiprone induced declines in proliferative responses at a dosage that is within peak serum pharmacological concentrations.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

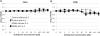
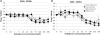
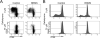
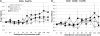
Similar articles
-
CD4+T-bet+, CD4+pSTAT3+ and CD8+T-bet+ T cells accumulate in peripheral blood during NZB treatment.Mult Scler. 2011 May;17(5):556-66. doi: 10.1177/1352458510392263. Epub 2010 Dec 21. Mult Scler. 2011. PMID: 21177324
-
Associations among IL-6 Signaling Molecules in Treg Populations in Patients with Relapsing-Remitting Multiple Sclerosis.J Integr Neurosci. 2025 May 26;24(5):36207. doi: 10.31083/JIN36207. J Integr Neurosci. 2025. PMID: 40464469
-
Differential effects of interferon-β1b on cytokine patterns of CD4+ and CD8+ T cells derived from RRMS and PPMS patients.Mult Scler. 2012 May;18(5):674-8. doi: 10.1177/1352458511427317. Epub 2011 Oct 24. Mult Scler. 2012. PMID: 22025329
-
[Interleukin-12 restores and promotes the T-cell immune function inhibited by 5-fluorouracil].Ai Zheng. 2007 Aug;26(8):801-8. Ai Zheng. 2007. PMID: 17697537 Chinese.
-
Increased in vitro induced CD4+ and CD8+ T cell IFN-gamma and CD4+ T cell IL-10 production in stable relapsing multiple sclerosis.Int J Neurosci. 1997 Aug;90(3-4):187-202. doi: 10.3109/00207459709000638. Int J Neurosci. 1997. PMID: 9352427 Review.
Cited by
-
Pathogenic implications of iron accumulation in multiple sclerosis.J Neurochem. 2012 Jan;120(1):7-25. doi: 10.1111/j.1471-4159.2011.07536.x. Epub 2011 Nov 11. J Neurochem. 2012. PMID: 22004421 Free PMC article. Review.
-
Rusty Microglia: Trainers of Innate Immunity in Alzheimer's Disease.Front Neurol. 2018 Dec 4;9:1062. doi: 10.3389/fneur.2018.01062. eCollection 2018. Front Neurol. 2018. PMID: 30564191 Free PMC article. Review.
-
Ferroptosis: A potential therapeutic target in autoimmune disease (Review).Exp Ther Med. 2023 Jun 15;26(2):368. doi: 10.3892/etm.2023.12067. eCollection 2023 Aug. Exp Ther Med. 2023. PMID: 37408857 Free PMC article. Review.
-
Iron chelation and multiple sclerosis.ASN Neuro. 2014 Jan 30;6(1):e00136. doi: 10.1042/AN20130037. ASN Neuro. 2014. PMID: 24397846 Free PMC article. Review.
-
Ferroptosis in immune chaos: Unraveling its impact on disease and therapeutic potential.J Physiol Biochem. 2025 May;81(2):249-272. doi: 10.1007/s13105-025-01078-7. Epub 2025 Apr 16. J Physiol Biochem. 2025. PMID: 40237936 Review.
References
-
- Sellebjerg F, Barnes D, Filippini G, Midgard R, Montalban X, Rieckmann P, et al. EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses. Eur J Neurol. 2005;12:939–46. - PubMed
-
- Myhr KM, Mellgren SI. Corticosteroids in the treatment of multiple sclerosis. Acta Neurol Scand Suppl. 2009;189:73–80. - PubMed
-
- Thrower BW. Relapse management in multiple sclerosis. Neurologist. 2009;15:1–5. Review. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

