Novel therapy to reverse the cellular effects of bisphosphonates on primary human oral fibroblasts
- PMID: 21807448
- PMCID: PMC3179815
- DOI: 10.1016/j.joms.2011.03.005
Novel therapy to reverse the cellular effects of bisphosphonates on primary human oral fibroblasts
Abstract
Purpose: Osteonecrosis of the jaws (ONJ) is a clinical condition that is characterized by a nonhealing breach in the oral mucosa resulting in exposure of bone and has been increasingly reported in patients receiving bisphosphonate (BP) therapy. Although the pathogenesis and natural history of ONJ remain ill-defined, it appears that the oral soft tissues play a critical role in the development of this condition. We examined the effects of the nitrogen-containing BPs pamidronate and zoledronate on primary human gingival fibroblasts.
Materials and methods: Primary gingival fibroblasts were exposed to clinically relevant doses of pamidronate and zoledronate. Cellular proliferation was measured with an MTS/PMS reagent-based kit (Promega, Madison, WI), scratch wound assays were performed to measure cellular migration, and apoptosis was measured by use of terminal deoxynucleotidyl transferase-mediated dUTP-FITC end labeling and caspase assays. The BP-exposed cells were treated with 10-ng/mL recombinant human platelet-derived growth factor BB (rhPDGF-BB) and 50-μmol/L geranylgeraniol (GGOH).
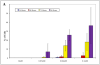
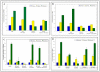
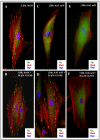
Results: Gingival fibroblasts are significantly more sensitive to inhibition of proliferation by zoledronate compared with pamidronate. Exposure of these cells to pamidronate but not zoledronate resulted in an increase in cellular apoptosis. Furthermore, exposure of gingival fibroblasts to pamidronate or zoledronate resulted in a decrease in cellular migration. We show that these defects are due to a loss of cell-substratum adhesion and a reduction of F-actin bundles. Finally, we show that the addition of rhPDGF-BB and GGOH in vitro is able to partially rescue the cell proliferation, migration, and adhesion defects.
Conclusion: The cytotoxic effects of BPs on oral fibroblasts and their significant reversal by the addition of GGOH and rhPDGF-BB provide both the potential mechanism and treatment options for ONJ.
Copyright © 2011 American Association of Oral and Maxillofacial Surgeons. Published by Elsevier Inc. All rights reserved.
Figures
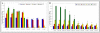




Comment in
-
Comments on Novel Therapy to Reverse the Cellular Effects of Bisphosphonates on Primary Human Oral Fibroblasts by Cozin M et al (2011).J Oral Maxillofac Surg. 2012 Jan;70(1):3. doi: 10.1016/j.joms.2011.10.021. J Oral Maxillofac Surg. 2012. PMID: 22182654 No abstract available.
References
-
- Colella G, Campisi G, Fusco V. American Association of Oral and Maxillofacial Surgeons position paper: Bisphosphonate-Related Osteonecrosis of the Jaws-2009 update: the need to refine the BRONJ definition. J Oral Maxillofac Surg. 2009;12:2698. - PubMed
-
- Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;10:1479. - PubMed
-
- Kyle RA, Yee GC, Somerfield MR, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;17:2464. - PubMed
-
- Patel V, McLeod NM, Rogers SN, et al. Bisphosphonate osteonecrosis of the jaw-a literature review of UK policies versus international policies on bisphosphonates, risk factors and prevention. Br J Oral Maxillofac Surg. 2010 - PubMed
-
- Ruggiero SL, Dodson TB, Assael LA, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;5(Suppl):2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

