Functionalized polystyrene supports for solid-phase synthesis of glycyl-, alanyl-, and isoleucyl-RNA conjugates as hydrolysis-resistant mimics of peptidyl-tRNAs
- PMID: 21807524
- PMCID: PMC3162138
- DOI: 10.1016/j.bmc.2011.07.018
Functionalized polystyrene supports for solid-phase synthesis of glycyl-, alanyl-, and isoleucyl-RNA conjugates as hydrolysis-resistant mimics of peptidyl-tRNAs
Abstract
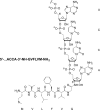
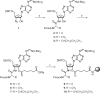
RNA-peptide conjugates that mimic amino acid-charged tRNAs and peptidyl-tRNAs are of high importance for structural and functional investigations of ribosomal complexes. Here, we present the synthesis of glycyl-, alanyl-, and isoleucyladenosine modified solid supports that are eligible for the synthesis of stable 3'-aminoacyl- and 3'-peptidyl-tRNA termini with an amide instead of the natural ester linkage. The present work significantly expands the range of accessible peptidyl-tRNA mimics for ribosomal studies.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
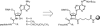

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

