Retinoic acid drives aryl hydrocarbon receptor expression and is instrumental to dioxin-induced toxicity during palate development
- PMID: 21807577
- PMCID: PMC3226489
- DOI: 10.1289/ehp.1003075
Retinoic acid drives aryl hydrocarbon receptor expression and is instrumental to dioxin-induced toxicity during palate development
Abstract
Background: Palate development depends on complex events and is very sensitive to disruption. Accordingly, clefts are the most common congenital malformations worldwide, and a connection is proposed with fetal exposure to toxic factors or environmental contaminants, such as dioxins. There is increasing evidence that dioxin interferes with all-trans-retinoic acid (atRA), a hormone-like signal derived from vitamin A, which plays an essential role during embryonic development. Although similarities have been described between dioxin-induced toxicity and the outcome of altered atRA signaling during palate development, their relationship needs to be clarified.
Objectives: We used a genetic approach to understand the interaction between atRA and dioxin and to identify the cell type targeted by dioxin toxicity during secondary palate formation in mice.
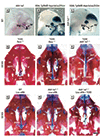
Methods: We analyzed the phenotype of mouse embryos harboring an atRA-sensitive reporter transgene or bearing null mutations for atRA-synthesizing enzymes (RALDH) or atRA receptors (RAR) and maternally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) at gestation day 10.5.
Results: We found that an intact atRA signal was required to enable TCDD to induce cleft palate. This mandatory atRA signal was generated through the activity of RALDH3 in the nasal epithelium and was transduced by RARγ (RARG) in the nasal mesenchyme, where it notably controlled aryl hydrocarbon receptor (Ahr) transcript levels. TCDD also did not alter the developmental pattern of atRA signaling during palate formation.
Conclusions: TCDD-induced alteration of secondary palate development in the mouse appears to depend on atRA signaling, which controls AHR expression. This mechanism is likely conserved throughout vertebrate evolution and may therefore be relevant in humans.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures




References
-
- Abbott BD, Birnbaum LS. TCDD-induced altered expression of growth factors may have a role in producing cleft palate and enhancing the incidence of clefts after coadministration of retinoic acid and TCDD. Toxicol Appl Pharmacol. 1990;106:418–432. - PubMed
-
- Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn. 1995;204:133–143. - PubMed
-
- Abbott BD, Harris MW, Birnbaum LS. Etiology of retinoic acid-induced cleft palate varies with the embryonic stage. Teratology. 1989;40:533–553. - PubMed
-
- Abbott BD, Held GA, Wood CR, Buckalew AR, Brown JG, Schmid J. Ahr, Arnt, and Cyp1a1 mRNA quantitation in cultured human embryonic palates exposed to TCDD and comparison with mouse palate in vivo and in culture. Toxicol Sci. 1999;47:62–75. - PubMed
-
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
