Thinking in cycles: MWC is a good model for acetylcholine receptor-channels
- PMID: 21807612
- PMCID: PMC3300048
- DOI: 10.1113/jphysiol.2011.214684
Thinking in cycles: MWC is a good model for acetylcholine receptor-channels
Abstract
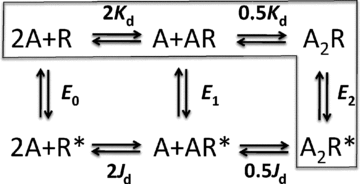
Neuromuscular acetylcholine receptors have long been a model system for understanding the mechanisms of operation of ligand-gated ion channels and fast chemical synapses. These five subunit membrane proteins have two allosteric (transmitter) binding sites and a distant ion channel domain. Occupation of the binding sites by agonist molecules transiently increases the probability that the channel is ion-permeable. Recent experiments show that the Monod, Wyman and Changeux formalism for allosteric proteins, originally developed for haemoglobin, is an excellent model for acetylcholine receptors. By using mutations and single-channel electrophysiology, the gating equilibrium constants for receptors with zero, one or two bound agonist molecules, and the agonist association and dissociation rate constants from both the closed- and open-channel conformations, have been estimated experimentally. The change in affinity for each transmitter molecule between closed and open conformations provides ~-5.1 kcal mol(-1) towards the global gating isomerization of the protein.
Figures




References
-
- Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. - PubMed
-
- Colquhoun D, Sakmann B. From muscle endplate to brain synapses: a short history of synapses and agonist-activated ion channels. Neuron. 1998;20:381–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

