Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids
- PMID: 21807613
- PMCID: PMC3213422
- DOI: 10.1113/jphysiol.2011.212845
Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids
Abstract
Skeletal muscle atrophy occurs under a variety of conditions and can result from alterations in both protein synthesis and protein degradation. The muscle-specific E3 ubiquitin ligases, MuRF1 and MAFbx, are excellent markers of muscle atrophy and increase under divergent atrophy-inducing conditions such as denervation and glucocorticoid treatment. While deletion of MuRF1 or MAFbx has been reported to spare muscle mass following 14 days of denervation, their role in other atrophy-inducing conditions is unclear. The goal of this study was to determine whether deletion of MuRF1 or MAFbx attenuates muscle atrophy after 2 weeks of treatment with the synthetic glucocorticoid dexamethasone (DEX). The response of the triceps surae (TS) and tibialis anterior (TA) muscles to 14 days of DEX treatment (3 mg kg(-1) day(-1)) was examined in 4 month-old male and female wild type (WT) and MuRF1 or MAFbx knock out (KO) mice. Following 14 days of DEX treatment, muscle wet weight was significantly decreased in the TS and TA of WT mice. Comparison of WT and KO mice following DEX treatment revealed significant sparing of mass in both sexes of the MuRF1 KO mice, but no muscle sparing in MAFbx KO mice. Further analysis of the MuRF1 KO mice showed significant sparing of fibre cross-sectional area and tension output in the gastrocnemius (GA) after DEX treatment. Muscle sparing in the MuRF1 KO mice was related to maintenance of protein synthesis, with no observed increases in protein degradation in either WT or MuRF1 KO mice. These results demonstrate that MuRF1 and MAFbx do not function similarly under all atrophy models, and that the primary role of MuRF1 may extend beyond controlling protein degradation via the ubiquitin proteasome system.
Figures
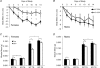
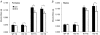
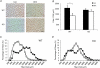
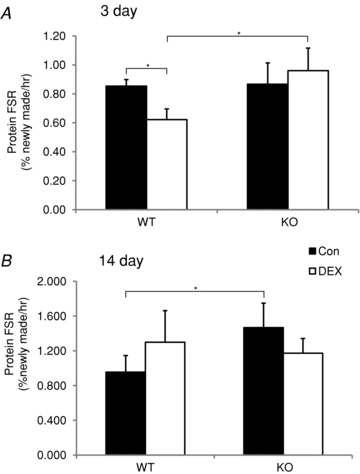
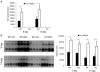
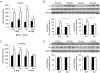
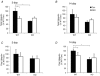
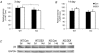
Comment in
-
Muscle atrophy; more than one string to MuRF1's bow?J Physiol. 2011 Oct 1;589(Pt 19):4635. doi: 10.1113/jphysiol.2011.218552. J Physiol. 2011. PMID: 21965626 Free PMC article. No abstract available.
References
-
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. - PubMed
-
- Auclair D, Garrel DR, Chaouki Zerouala A, Ferland LH. Activation of the ubiquitin pathway in rat skeletal muscle by catabolic doses of glucocorticoids. Am J Physiol Cell Physiol. 1997;272:C1007–C1016. - PubMed
-
- Barry SC, Gallagher CG. Corticosteroids and skeletal muscle function in cystic fibrosis. J Appl Physiol. 2003;95:1379–1384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

