Low-fluence red light increases the transport and biosynthesis of auxin
- PMID: 21807888
- PMCID: PMC3192557
- DOI: 10.1104/pp.111.181388
Low-fluence red light increases the transport and biosynthesis of auxin
Abstract
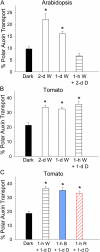
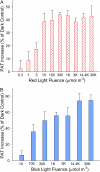
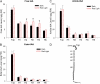
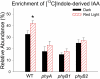
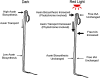
In plants, light is an important environmental signal that induces photomorphogenesis and interacts with endogenous signals, including hormones. We found that light increased polar auxin transport in dark-grown Arabidopsis (Arabidopsis thaliana) and tomato (Solanum lycopersicum) hypocotyls. In tomato, this increase was induced by low-fluence red or blue light followed by 1 d of darkness. It was reduced in phyA, phyB1, and phyB2 tomato mutants and was reversed by far-red light applied immediately after the red or blue light exposure, suggesting that phytochrome is involved in this response. We further found that the free indole-3-acetic acid (IAA) level in hypocotyl regions below the hook was increased by red light, while the level of conjugated IAA was unchanged. Analysis of IAA synthesized from [¹³C]indole or [¹³C]tryptophan (Trp) revealed that both Trp-dependent and Trp-independent IAA biosynthesis were increased by low-fluence red light in the top section (meristem, cotyledons, and hook), and the Trp-independent pathway appears to become the primary route for IAA biosynthesis after red light exposure. IAA biosynthesis in tissues below the top section was not affected by red light, suggesting that the increase of free IAA in this region was due to increased transport of IAA from above. Our study provides a comprehensive view of light effects on the transport and biosynthesis of IAA, showing that red light increases both IAA biosynthesis in the top section and polar auxin transport in hypocotyls, leading to unchanged free IAA levels in the top section and increased free IAA levels in the lower hypocotyl regions.
Figures





Similar articles
-
Enhancement of hypocotyl elongation by LOV KELCH PROTEIN2 production is mediated by auxin and phytochrome-interacting factors in Arabidopsis thaliana.Plant Cell Rep. 2016 Feb;35(2):455-67. doi: 10.1007/s00299-015-1896-4. Epub 2015 Nov 25. Plant Cell Rep. 2016. PMID: 26601822
-
Complexity of the auxin biosynthetic network in Arabidopsis hypocotyls is revealed by multiple stable-labeled precursors.Phytochemistry. 2022 Aug;200:113219. doi: 10.1016/j.phytochem.2022.113219. Epub 2022 May 4. Phytochemistry. 2022. PMID: 35523282
-
Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis.Plant Physiol. 1998 Feb;116(2):455-62. doi: 10.1104/pp.116.2.455. Plant Physiol. 1998. PMID: 9489005 Free PMC article.
-
Current aspects of auxin biosynthesis in plants.Biosci Biotechnol Biochem. 2016;80(1):34-42. doi: 10.1080/09168451.2015.1086259. Epub 2015 Sep 12. Biosci Biotechnol Biochem. 2016. PMID: 26364770 Review.
-
Auxin: regulation, action, and interaction.Ann Bot. 2005 Apr;95(5):707-35. doi: 10.1093/aob/mci083. Epub 2005 Mar 4. Ann Bot. 2005. PMID: 15749753 Free PMC article. Review.
Cited by
-
AUXIN-BINDING-PROTEIN1 (ABP1) in phytochrome-B-controlled responses.J Exp Bot. 2013 Nov;64(16):5065-74. doi: 10.1093/jxb/ert294. Epub 2013 Sep 19. J Exp Bot. 2013. PMID: 24052532 Free PMC article.
-
Arabidopsis Phytochrome A Directly Targets Numerous Promoters for Individualized Modulation of Genes in a Wide Range of Pathways.Plant Cell. 2014 May;26(5):1949-1966. doi: 10.1105/tpc.114.123950. Epub 2014 May 2. Plant Cell. 2014. PMID: 24794133 Free PMC article.
-
A role for the root cap in root branching revealed by the non-auxin probe naxillin.Nat Chem Biol. 2012 Sep;8(9):798-805. doi: 10.1038/nchembio.1044. Epub 2012 Aug 12. Nat Chem Biol. 2012. PMID: 22885787 Free PMC article.
-
Effects of rare-earth light conversion film on the growth and fruit quality of sweet pepper in a solar greenhouse.Front Plant Sci. 2022 Sep 6;13:989271. doi: 10.3389/fpls.2022.989271. eCollection 2022. Front Plant Sci. 2022. PMID: 36147241 Free PMC article.
-
Shedding light on auxin movement: light-regulation of polar auxin transport in the photocontrol of plant development.Plant Signal Behav. 2013 Mar;8(3):e23355. doi: 10.4161/psb.23355. Epub 2013 Jan 18. Plant Signal Behav. 2013. PMID: 23333970 Free PMC article. Review.
References
-
- Aloni R. (2010) The induction of vascular tissues by auxin. Davies P, , Plant Hormones, Ed 3. Springer, Dordrecht, The Netherlands, pp 485–518
-
- Bandurski RS, Schulze A, Cohen JD. (1977) Photo-regulation of the ratio of ester to free indole-3-acetic acid. Biochem Biophys Res Commun 79: 1219–1223 - PubMed
-
- Barkawi LS, Tam Y-Y, Tillman JA, Normanly J, Cohen JD. (2010) A high-throughput method for the quantitative analysis of auxins. Nat Protoc 5: 1609–1618 - PubMed
-
- Barker-Bridges M, Ribnicky D, Cohen J, Jones A. (1998) Red light-regulated growth. II. Changes in the abundance of indoleacetic acid in the maize mesocotyl. Planta 204: 207–211
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

