Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection
- PMID: 21807912
- PMCID: PMC3187263
- DOI: 10.1128/IAI.05493-11
Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection
Abstract
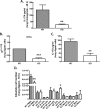
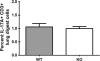
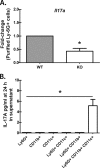
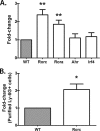
We have previously reported that compromised interleukin 17A (IL-17A) production in the lungs increased susceptibility to infection with the invasive fungal pathogen Aspergillus fumigatus. Here we have shown that culturing lung cells from A. fumigatus-challenged mice ex vivo demonstrated Dectin-1-dependent IL-17A production. In this system, neutralization of IL-23 but not IL-6, IL-1β, or IL-18 resulted in attenuated IL-17A production. Il23 mRNA expression was found to be lower in lung cells from A. fumigatus-challenged Dectin-1-deficient mice, whereas bone marrow-derived dendritic cells from Dectin-1-deficient mice failed to produce IL-23 in response to A. fumigatus in vitro. Addition of recombinant IL-23 augmented IL-17A production by wild-type (WT) and Dectin-1-deficient lung cells, although the addition of IL-6 or IL-1β did not augment the effect of IL-23. Intracellular cytokine staining of lung cells revealed lower levels of CD11b(+) IL-17A(+) and Ly-6G(+) IL-17A(+) cells in A. fumigatus-challenged Dectin-1-deficient mice. Ly-6G(+) neutrophils purified from the lungs of A. fumigatus-challenged Dectin-1-deficient mice displayed lower Il17a mRNA expression but surprisingly had intact Rorc and Rora mRNA expression. We further demonstrated that Ly-6G(+) neutrophils required the presence of myeloid cells for IL-17A production. Finally, upon in vitro stimulation with A. fumigatus, thioglycolate-elicited peritoneal neutrophils were positive for intracellular IL-17A expression and produced IL-17A in a Dectin-1- and IL-23-dependent manner. In summary, Dectin-1-dependent IL-17A production in the lungs during invasive fungal infection is mediated in part by CD11b(+) Ly-6G(+) neutrophils in an IL-23-dependent manner.
Figures


References
-
- Baddley J. W., Stroud T. P., Salzman D., Pappas P. G. 2001. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 32:1319–1324 - PubMed
-
- Barnes P. D., Marr K. A. 2007. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br. J. Haematol. 139:519–531 - PubMed
-
- Brustle A., et al. 2007. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 8:958–966 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

