Suppression of autophagy by FIP200 deletion impairs DNA damage repair and increases cell death upon treatments with anticancer agents
- PMID: 21807966
- PMCID: PMC3175275
- DOI: 10.1158/1541-7786.MCR-11-0098
Suppression of autophagy by FIP200 deletion impairs DNA damage repair and increases cell death upon treatments with anticancer agents
Abstract
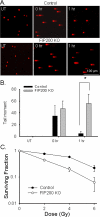
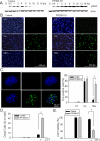
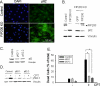
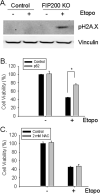
Autophagy is a lysosomal bulk degradation process for intracellular protein and organelles. FIP200 (200 kDa FAK-family interacting protein) is an essential component of mammalian autophagy that is implicated in breast cancer in recent studies. Here we show that inactivation of FIP200 resulted in deficient repair of DNA damage induced by ionizing radiation and anticancer agents in mouse embryonic fibroblasts (MEF). The persistent DNA damage correlated to increased apoptosis and reduced survival of FIP200 knockout (KO) MEFs after treatments with camptothecin (CPT), a topoisomerase I inhibitor and chemotherapeutic agent. Reexpression of FIP200 in FIP200 KO MEFs restored both efficient DNA damage repair and cell survival. Furthermore, knockdown of the increased p62 expression in FIP200 KO MEFs rescued the impaired DNA damage repair and CPT-induced cell death. In contrast, treatment of cells with N-acetyl cysteine did not affect these defects in FIP200 KO MEFs. Finally, FIP200 KO MEFs also showed deficient DNA damage repair and increased cell death compared with control MEFs, when treated with etoposide, a topoisomerase II inhibitor and another anticancer agent. Together, these results identify a new function for FIP200 in the regulation of DNA damage response and cell survival through its activity in autophagy and suggest the possibility of FIP200 or other autophagy proteins as a potential target for treatment to enhance the efficiency of cancer therapy using DNA damage-inducing agents.
©2011 AACR.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

