Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization
- PMID: 21807983
- PMCID: PMC3186970
- DOI: 10.1128/AAC.00182-11
Pharmacokinetic/pharmacodynamic (PK/PD) indices of antibiotics predicted by a semimechanistic PKPD model: a step toward model-based dose optimization
Abstract
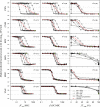
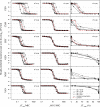
A pharmacokinetic-pharmacodynamic (PKPD) model that characterizes the full time course of in vitro time-kill curve experiments of antibacterial drugs was here evaluated in its capacity to predict the previously determined PK/PD indices. Six drugs (benzylpenicillin, cefuroxime, erythromycin, gentamicin, moxifloxacin, and vancomycin), representing a broad selection of mechanisms of action and PK and PD characteristics, were investigated. For each drug, a dose fractionation study was simulated, using a wide range of total daily doses given as intermittent doses (dosing intervals of 4, 8, 12, or 24 h) or as a constant drug exposure. The time course of the drug concentration (PK model) as well as the bacterial response to drug exposure (in vitro PKPD model) was predicted. Nonlinear least-squares regression analyses determined the PK/PD index (the maximal unbound drug concentration [fC(max)]/MIC, the area under the unbound drug concentration-time curve [fAUC]/MIC, or the percentage of a 24-h time period that the unbound drug concentration exceeds the MIC [fT(>MIC)]) that was most predictive of the effect. The in silico predictions based on the in vitro PKPD model identified the previously determined PK/PD indices, with fT(>MIC) being the best predictor of the effect for β-lactams and fAUC/MIC being the best predictor for the four remaining evaluated drugs. The selection and magnitude of the PK/PD index were, however, shown to be sensitive to differences in PK in subpopulations, uncertainty in MICs, and investigated dosing intervals. In comparison with the use of the PK/PD indices, a model-based approach, where the full time course of effect can be predicted, has a lower sensitivity to study design and allows for PK differences in subpopulations to be considered directly. This study supports the use of PKPD models built from in vitro time-kill curves in the development of optimal dosing regimens for antibacterial drugs.
Figures

References
-
- Ambrose P. G., et al. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 - PubMed
-
- Andes D., Craig W. A. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19:261–268 - PubMed
-
- Barbour A., Scaglione F., Derendorf H. 2010. Class-dependent relevance of tissue distribution in the interpretation of anti-infective pharmacokinetic/pharmacodynamic indices. Int. J. Antimicrob. Agents 35:431–438 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

