Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators
- PMID: 21807990
- PMCID: PMC3198917
- DOI: 10.1124/mol.111.073239
Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators
Abstract
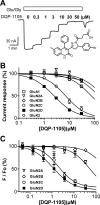
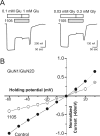
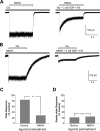
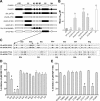
The compound 4-(5-(4-bromophenyl)-3-(6-methyl-2-oxo-4-phenyl-1,2-dihydroquinolin-3-yl)-4,5-dihydro-1H-pyrazol-1-yl)-4-oxobutanoic acid (DQP-1105) is a representative member of a new class of N-methyl-d-aspartate (NMDA) receptor antagonists. DQP-1105 inhibited GluN2C- and GluN2D-containing receptors with IC(50) values that were at least 50-fold lower than those for recombinant GluN2A-, GluN2B-, GluA1-, or GluK2-containing receptors. Inhibition was voltage-independent and could not be surmounted by increasing concentrations of either coagonist, glutamate or glycine, consistent with a noncompetitive mechanism of action. DQP-1105 inhibited single-channel currents in excised outside-out patches without significantly changing mean open time or single-channel conductance, suggesting that DQP inhibits a pregating step without changing the stability of the open pore conformation and thus channel closing rate. Evaluation of DQP-1105 inhibition of chimeric NMDA receptors identified two key residues in the lower lobe of the GluN2 agonist binding domain that control the selectivity of DQP-1105. These data suggest a mechanism for this new class of inhibitors and demonstrate that ligands can access, in a subunit-selective manner, a new site located in the lower, membrane-proximal portion of the agonist-binding domain.
Figures




References
-
- Chen HS, Lipton SA. (2006) The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem 97:1611–1626 - PubMed
-
- Colquhoun D, Sigworth FJ. (1995) Fitting and Statistical Analysis of Single-Channel Recording, Plenum Press, New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

