Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy
- PMID: 21808006
- PMCID: PMC3158220
- DOI: 10.1073/pnas.1016778108
Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy
Abstract
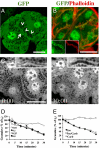
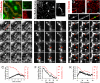
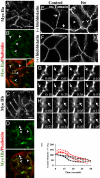
The regulation and the dynamics of membrane trafficking events have been studied primarily in in vitro models that often do not fully reflect the functional complexity found in a living multicellular organism. Here we used intravital microscopy in the salivary glands of live rodents to investigate regulated exocytosis, a fundamental process in all of the secretory organs. We found that β-adrenergic stimulation elicits exocytosis of large secretory granules, which gradually collapse with the apical plasma membrane without any evidence of compound exocytosis, as was previously described. Furthermore, we show that the driving force required to complete the collapse of the granules is provided by the recruitment of F-actin and nonmuscle myosin II on the granule membranes that is triggered upon fusion with the plasma membrane. Our results provide information on the machinery controlling regulated secretion and show that intravital microscopy provides unique opportunities to address fundamental questions in cell biology under physiological conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. - PubMed
-
- De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. - PubMed
-
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. - PubMed
-
- Castle AM, Huang AY, Castle JD. The minor regulated pathway, a rapid component of salivary secretion, may provide docking/fusion sites for granule exocytosis at the apical surface of acinar cells. J Cell Sci. 2002;115:2963–2973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

