Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells
- PMID: 21808010
- PMCID: PMC3158209
- DOI: 10.1073/pnas.1107172108
Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells
Abstract
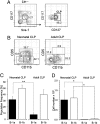
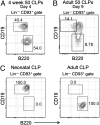
B-1 B cells have been proposed to be preferentially generated from fetal progenitors, but this view is challenged by studies concluding that B-1 production is sustained throughout adult life. To address this controversy, we compared the efficiency with which hematopoietic stem cells (HSCs) and common lymphoid progenitors (CLPs) from neonates and adults generated B-1 cells in vivo and developed a clonal in vitro assay to quantify B-1 progenitor production from CLPs. Adult HSCs and CLPs generated fewer B-1 cells in vivo compared with their neonatal counterparts, a finding corroborated by the clonal studies that showed that the CLP compartment includes B-1- and B-2-specified subpopulations and that the former cells decrease in number after birth. Together, these data indicate that B-1 lymphopoiesis is not sustained at constant levels throughout life and define a heretofore unappreciated developmental heterogeneity within the CLP compartment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. - PubMed
-
- Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. - PubMed
-
- Herzenberg LA, Tung JW. B cell lineages: Documented at last! Nat Immunol. 2006;7:225–226. - PubMed
-
- Alugupalli KR, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

