The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus
- PMID: 21808016
- PMCID: PMC3158184
- DOI: 10.1073/pnas.1105115108
The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus
Abstract
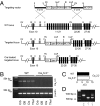
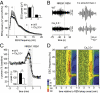
Low-threshold (T-type) Ca(2+) channels encoded by the Ca(V)3 genes endow neurons with oscillatory properties that underlie slow waves characteristic of the non-rapid eye movement (NREM) sleep EEG. Three Ca(V)3 channel subtypes are expressed in the thalamocortical (TC) system, but their respective roles for the sleep EEG are unclear. Ca(V)3.3 protein is expressed abundantly in the nucleus reticularis thalami (nRt), an essential oscillatory burst generator. We report the characterization of a transgenic Ca(V)3.3(-/-) mouse line and demonstrate that Ca(V)3.3 channels are indispensable for nRt function and for sleep spindles, a hallmark of natural sleep. The absence of Ca(V)3.3 channels prevented oscillatory bursting in the low-frequency (4-10 Hz) range in nRt cells but spared tonic discharge. In contrast, adjacent TC neurons expressing Ca(V)3.1 channels retained low-threshold bursts. Nevertheless, the generation of synchronized thalamic network oscillations underlying sleep-spindle waves was weakened markedly because of the reduced inhibition of TC neurons via nRt cells. T currents in Ca(V)3.3(-/-) mice were <30% compared with those in WT mice, and the remaining current, carried by Ca(V)3.2 channels, generated dendritic [Ca(2+)](i) signals insufficient to provoke oscillatory bursting that arises from interplay with Ca(2+)-dependent small conductance-type 2 K(+) channels. Finally, naturally sleeping Ca(V)3.3(-/-) mice showed a selective reduction in the power density of the σ frequency band (10-12 Hz) at transitions from NREM to REM sleep, with other EEG waves remaining unaltered. Together, these data identify a central role for Ca(V)3.3 channels in the rhythmogenic properties of the sleep-spindle generator and provide a molecular target to elucidate the roles of sleep spindles for brain function and development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. - PubMed
-
- Klöckner U, et al. Comparison of the Ca2+ currents induced by expression of three cloned α1 subunits, α1G, α1H and α1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci. 1999;11:4171–4178. - PubMed
-
- Broicher T, Kanyshkova T, Meuth P, Pape HC, Budde T. Correlation of T-channel coding gene expression, IT, and the low threshold Ca2+ spike in the thalamus of a rat model of absence epilepsy. Mol Cell Neurosci. 2008;39:384–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

