Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function
- PMID: 21808020
- PMCID: PMC3158170
- DOI: 10.1073/pnas.1111093108
Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function
Abstract
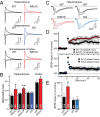
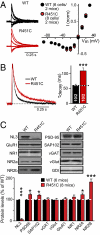
Multiple independent mutations in neuroligin genes were identified in patients with familial autism, including the R451C substitution in neuroligin-3 (NL3). Previous studies showed that NL3(R451C) knock-in mice exhibited modestly impaired social behaviors, enhanced water maze learning abilities, and increased synaptic inhibition in the somatosensory cortex, and they suggested that the behavioral changes in these mice may be caused by a general shift of synaptic transmission to inhibition. Here, we confirm that NL3(R451C) mutant mice behaviorally exhibit social interaction deficits and electrophysiologically display increased synaptic inhibition in the somatosensory cortex. Unexpectedly, however, we find that the NL3(R451C) mutation produced a strikingly different phenotype in the hippocampus. Specifically, in the hippocampal CA1 region, the NL3(R451C) mutation caused an ∼1.5-fold increase in AMPA receptor-mediated excitatory synaptic transmission, dramatically altered the kinetics of NMDA receptor-mediated synaptic responses, induced an approximately twofold up-regulation of NMDA receptors containing NR2B subunits, and enhanced long-term potentiation almost twofold. NL3 KO mice did not exhibit any of these changes. Quantitative light microscopy and EM revealed that the NL3(R451C) mutation increased dendritic branching and altered the structure of synapses in the stratum radiatum of the hippocampus. Thus, in NL3(R451C) mutant mice, a single point mutation in a synaptic cell adhesion molecule causes context-dependent changes in synaptic transmission; these changes are consistent with the broad impact of this mutation on murine and human behaviors, suggesting that NL3 controls excitatory and inhibitory synapse properties in a region- and circuit-specific manner.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- American Psychiatric Association . Diagnostic Criteria from DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
-
- Folstein S, Rutter M. Infantile autism: A genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18:297–321. - PubMed
-
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: Genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

