Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading
- PMID: 21808040
- PMCID: PMC3167546
- DOI: 10.1073/pnas.1105845108
Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading
Abstract
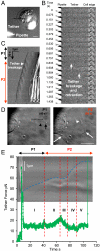
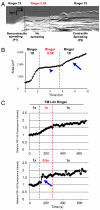
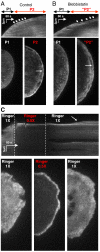
Cell migration and spreading involve the coordination of membrane trafficking, actomyosin contraction, and modifications to plasma membrane tension and area. The biochemical or biophysical basis for this coordination is however unknown. In this study, we show that during cell spreading, lamellipodia protrusion flattens plasma membrane folds and blebs and, once the plasma membrane area is depleted, there is a temporary increase in membrane tension by over twofold that is followed by activation of exocytosis and myosin contraction. Further, an artificial increase in plasma membrane tension stopped lamellipodia protrusion and activated an exocytotic burst. Subsequent decrease in tension restored spreading with activation of contraction. Conversely, blebbistatin inhibition of actomyosin contraction resulted in an even greater increase in plasma membrane tension and exocytosis activation. This spatiotemporal synchronization indicates that membrane tension is the signal that coordinates membrane trafficking, actomyosin contraction, and plasma membrane area change. We suggest that cells use plasma membrane tension as a global physical parameter to control cell motility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Membrane tension leads the way.Proc Natl Acad Sci U S A. 2011 Aug 30;108(35):14379-80. doi: 10.1073/pnas.1111671108. Epub 2011 Aug 23. Proc Natl Acad Sci U S A. 2011. PMID: 21873200 Free PMC article. No abstract available.
References
-
- Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol. 2002;282:F179–190. - PubMed
-
- Morris CE, Homann U. Cell surface area regulation and membrane tension. J Membr Biol. 2001;179:79–102. - PubMed
-
- Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2:392–396. - PubMed
-
- Sheetz MP, Sable JE, Dobereiner HG. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct. 2006;35:417–434. - PubMed
-
- Kell A, Glaser RW. On the mechanical and dynamic properties of plant cell membranes: Their role in growth, direct gene transfer and protoplast fusion. J Theor Biol. 1993;160:41–62.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

