A bacterial process for selenium nanosphere assembly
- PMID: 21808043
- PMCID: PMC3158160
- DOI: 10.1073/pnas.1105959108
A bacterial process for selenium nanosphere assembly
Abstract
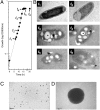
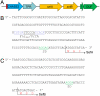
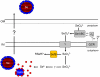
During selenate respiration by Thauera selenatis, the reduction of selenate results in the formation of intracellular selenium (Se) deposits that are ultimately secreted as Se nanospheres of approximately 150 nm in diameter. We report that the Se nanospheres are associated with a protein of approximately 95 kDa. Subsequent experiments to investigate the expression and secretion profile of this protein have demonstrated that it is up-regulated and secreted in response to increasing selenite concentrations. The protein was purified from Se nanospheres, and peptide fragments from a tryptic digest were used to identify the gene in the draft T. selenatis genome. A matched open reading frame was located, encoding a protein with a calculated mass of 94.5 kDa. N-terminal sequence analysis of the mature protein revealed no cleavable signal peptide, suggesting that the protein is exported directly from the cytoplasm. The protein has been called Se factor A (SefA), and homologues of known function have not been reported previously. The sefA gene was cloned and expressed in Escherichia coli, and the recombinant His-tagged SefA purified. In vivo experiments demonstrate that SefA forms larger (approximately 300 nm) Se nanospheres in E. coli when treated with selenite, and these are retained within the cell. In vitro assays demonstrate that the formation of Se nanospheres upon the reduction of selenite by glutathione are stabilized by the presence of SefA. The role of SefA in selenium nanosphere assembly has potential for exploitation in bionanomaterial fabrication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Richardson DJ. Bacterial respiration: A flexible process for a changing environment. Microbiology. 2000;146:551–571. - PubMed
-
- Lloyd JR. Microbial reduction of metals and radionuclides. FEMS Microbiol Rev. 2003;27:411–425. - PubMed
-
- Macy JM, et al. Thauera selenatis gen-nov, sp-nov, a member of the beta-subclass of proteobacteria with a novel type of anaerobic respiration. Int J Syst Bacteriol. 1993;43:135–142. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

