Regulation of multiple carbon monoxide consumption pathways in anaerobic bacteria
- PMID: 21808633
- PMCID: PMC3135865
- DOI: 10.3389/fmicb.2011.00147
Regulation of multiple carbon monoxide consumption pathways in anaerobic bacteria
Erratum in
-
Corrigendum: Regulation of multiple carbon monoxide consumption pathways in anaerobic bacteria.Front Microbiol. 2018 Jul 5;9:1016. doi: 10.3389/fmicb.2018.01016. eCollection 2018. Front Microbiol. 2018. PMID: 30013517 Free PMC article.
Abstract
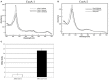
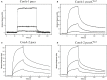
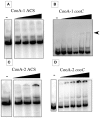
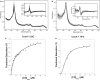
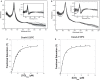
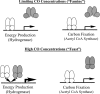
Carbon monoxide (CO), well known as a toxic gas, is increasingly recognized as a key metabolite and signaling molecule. Microbial utilization of CO is quite common, evidenced by the rapid escalation in description of new species of CO-utilizing bacteria and archaea. Carbon monoxide dehydrogenase (CODH), the protein complex that enables anaerobic CO-utilization, has been well-characterized from an increasing number of microorganisms, however the regulation of multiple CO-related gene clusters in single isolates remains unexplored. Many species are extraordinarily resistant to high CO concentrations, thriving under pure CO at more than one atmosphere. We hypothesized that, in strains that can grow exclusively on CO, both carbon acquisition via the CODH/acetyl CoA synthase complex and energy conservation via a CODH-linked hydrogenase must be differentially regulated in response to the availability of CO. The CO-sensing transcriptional activator, CooA is present in most CO-oxidizing bacteria. Here we present a genomic and phylogenetic survey of CODH operons and cooA genes found in CooA-containing bacteria. Two distinct groups of CooA homologs were found: one clade (CooA-1) is found in the majority of CooA-containing bacteria, whereas the other clade (CooA-2) is found only in genomes that encode multiple CODH clusters, suggesting that the CooA-2 might be important for cross-regulation of competing CODH operons. Recombinant CooA-1 and CooA-2 regulators from the prototypical CO-utilizing bacterium Carboxydothermus hydrogenoformans were purified, and promoter binding analyses revealed that CooA-1 specifically regulates the hydrogenase-linked CODH, whereas CooA-2 is able to regulate both the hydrogenase-linked CODH and the CODH/ACS operons. These studies point to the ability of dual CooA homologs to partition CO into divergent CO-utilizing pathways resulting in efficient consumption of a single limiting growth substrate available across a wide range of concentrations.
Keywords: Carboxydothermus hydrogenoformans; CooA; carbon monoxide; carbon monoxide dehydrogenase; carboxydotrophs; hydrogenogens; thermophiles.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

