Unique mode of lipogenic activation in rat preputial sebocytes
- PMID: 21808727
- PMCID: PMC3144693
- DOI: 10.1155/2011/163631
Unique mode of lipogenic activation in rat preputial sebocytes
Abstract
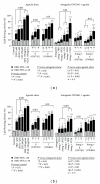
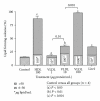
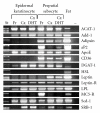
Lipoprotein delivery of fatty acids and cholesterol is linked with peroxisome proliferator-activated receptor (PPAR) activation in adipocytes and macrophages. We postulated that similar interactions exist in sebaceous epithelial cells (sebocytes) in which PPAR activation induces differentiation. High-density lipoprotein (HDL) and very low-density lipoprotein (VLDL) markedly enhanced sebocyte differentiation above that found with PPAR agonists and were more potent than explicable by their lipid content. The PPARγ antagonist GW5393 reduced sebocyte differentiation to all PPAR isoform agonists, HDL and VLDL, suggesting that the lipoprotein effect on differentiation occurs partially through activation of PPARγ. Furthermore, we found that sebocytes expressed a unique pattern of lipogenic genes. Our results demonstrate that HDL and VLDL are the most potent inducers of sebocyte differentiation tested to date, and these actions are partially inhibited by PPAR antagonists. This suggests that substrates provided by lipoproteins are targeted to sebocytes and affect their own disposition via PPAR activation.
Figures

Similar articles
-
Rat preputial sebocyte differentiation involves peroxisome proliferator-activated receptors.J Invest Dermatol. 1999 Feb;112(2):226-32. doi: 10.1046/j.1523-1747.1999.00487.x. J Invest Dermatol. 1999. PMID: 9989800
-
Limited cooperation between peroxisome proliferator-activated receptors and retinoid X receptor agonists in sebocyte growth and development.Mol Genet Metab. 2001 Nov;74(3):362-9. doi: 10.1006/mgme.2001.3242. Mol Genet Metab. 2001. PMID: 11708867
-
Sox9 facilitates proliferation, differentiation and lipogenesis in primary cultured human sebocytes.J Dermatol Sci. 2017 Jan;85(1):44-50. doi: 10.1016/j.jdermsci.2016.10.005. Epub 2016 Oct 13. J Dermatol Sci. 2017. PMID: 27771230
-
Peroxisome proliferator-activated receptors and skin development.Horm Res. 2000;54(5-6):269-74. doi: 10.1159/000053270. Horm Res. 2000. PMID: 11595816 Review.
-
Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression.J Lipid Res. 1996 May;37(5):907-25. J Lipid Res. 1996. PMID: 8725145 Review.
Cited by
-
Total cholesterol and lipoprotein composition are associated with dry eye disease in Korean women.Lipids Health Dis. 2013 Jun 5;12:84. doi: 10.1186/1476-511X-12-84. Lipids Health Dis. 2013. PMID: 23734839 Free PMC article.
-
Relationship between Dry Eye Disease and Dyslipidemia: A Systematic Review.J Clin Med. 2023 Oct 20;12(20):6631. doi: 10.3390/jcm12206631. J Clin Med. 2023. PMID: 37892769 Free PMC article. Review.
-
Seed Watermelon (Citrullus mucosospermus (Fursa))-Derived Coniferyl Alcohol as a Functional Ingredient in Remedies for Dry Skin: Evidence of Facilitated Lipogenesis in Human Sebocytes.Molecules. 2025 Aug 13;30(16):3360. doi: 10.3390/molecules30163360. Molecules. 2025. PMID: 40871513 Free PMC article.
-
Dyslipidemia and its Association with Meibomian Gland Dysfunction: A Systematic Review.Int Ophthalmol. 2018 Aug;38(4):1809-1816. doi: 10.1007/s10792-017-0633-0. Epub 2017 Jul 7. Int Ophthalmol. 2018. PMID: 28688025
References
-
- Rosenfield RL, Kentsis A, Deplewski D, Ciletti N. Rat preputial sebocyte differentiation involves peroxisome proliferator-activated receptors. Journal of Investigative Dermatology. 1999;112(2):226–232. - PubMed
-
- Chen W, Yang CC, Sheu HM, Seltmann H, Zouboulis CC. Expression of peroxisome proliferator-activated receptor and CCAAT/enhancer binding protein transcription factors in cultured human sebocytes. Journal of Investigative Dermatology. 2003;121(3):441–447. - PubMed
-
- Akimoto N, Sato T, Iwata C, et al. Expression of perilipin A on the surface of lipid droplets increases along with the differentiation of hamster sebocytes in vivo and in vitro. Journal of Investigative Dermatology. 2005;124(6):1127–1133. - PubMed
-
- Makrantonaki E, Zouboulis CC. Testosterone metabolism to 5α-dihydrotestosterone and synthesis of sebaceous lipids is regulated by the peroxisome proliferator-activated receptor ligand linoleic acid in human sebocytes. British Journal of Dermatology. 2007;156(3):428–432. - PubMed
-
- Downie MM, Sanders DA, Maier LM, Stock DM, Kealey T. Peroxisome proliferator-activated receptor and farnesoid X receptor ligands differentially regulate sebaceous differentiation in human sebaceous gland organ cultures in vitro. British Journal of Dermatology. 2004;151(4):766–775. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

