Quality assessment of HER2 testing by monitoring of positivity rates
- PMID: 21809092
- PMCID: PMC3162627
- DOI: 10.1007/s00428-011-1132-8
Quality assessment of HER2 testing by monitoring of positivity rates
Abstract
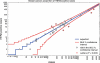
Interlaboratory variation in human epidermal growth factor receptor 2 (HER2) testing provides a challenge for targeted therapy in breast and gastric cancer. Assessment of positivity rates among laboratories could help monitor their performance and define reference values for positivity rates to be expected in a geographic region. Pathologists regularly determined the number of HER2-positive cases (HER2 3+, HER2 2+/amplified or amplified) in their laboratory, and figures were continuously entered into a central website. The overall positivity rate of each participant was calculated and compared with the average rates of all other institutes (n = 42). A total of 18,081 test results on breast cancer and 982 on gastric cancer were entered into the system. Positivity rates for HER2 in breast cancer ranged from 7.6% to 31.6%. Statistically, the results from six institutions qualified as outliers (p < 0.000005). From the remaining institutions encompassing 10,916 assessments, the mean proportion of positive cases was 16.7 ± 3.2% (99% confidence interval 16.6-16.8). The results from six institutions were in between the 95% and 99.5% confidence intervals. For gastric cancer, there was one outlier and the mean positivity rate was 23.2 ± 5.7%. The proportion of HER2-positive breast cancer cases is considerably lower than could have been expected from published studies. By assessing the positivity rates and comparing them with that of all breast or gastric cancers in a given population, pathologists will be alerted to a potential systematic error in their laboratory assay, causative for over- or underestimation of cancer cases suited for anti-HER2 therapy.
Figures


References
-
- Paik S, Bryant J, Tan-Chiu E, Romond E, Hiller W, et al. Real-world performance of HER2 testing—National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;94:852–854. - PubMed
-
- Roche PC, Suman VJ, Jenkins RB, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst. 2002;94:855–857. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

