A review of the mammalian unfolded protein response
- PMID: 21809331
- PMCID: PMC3193940
- DOI: 10.1002/bit.23282
A review of the mammalian unfolded protein response
Abstract
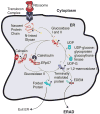
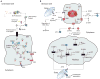
Proteins requiring post-translational modifications such as N-linked glycosylation are processed in the endoplasmic reticulum (ER). A diverse array of cellular stresses can lead to dysfunction of the ER and ultimately to an imbalance between protein-folding capacity and protein-folding load. Cells monitor protein folding by an inbuilt quality control system involving both the ER and the Golgi apparatus. Unfolded or misfolded proteins are tagged for degradation via ER-associated degradation (ERAD) or sent back through the folding cycle. Continued accumulation of incorrectly folded proteins can also trigger the unfolded protein response (UPR). In mammalian cells, UPR is a complex signaling program mediated by three ER transmembrane receptors: activating transcription factor 6 (ATF6), inositol requiring kinase 1 (IRE1) and double-stranded RNA-activated protein kinase (PKR)-like endoplasmic reticulum kinase (PERK). UPR performs three functions, adaptation, alarm, and apoptosis. During adaptation, the UPR tries to reestablish folding homeostasis by inducing the expression of chaperones that enhance protein folding. Simultaneously, global translation is attenuated to reduce the ER folding load while the degradation rate of unfolded proteins is increased. If these steps fail, the UPR induces a cellular alarm and mitochondrial mediated apoptosis program. UPR malfunctions have been associated with a wide range of disease states including tumor progression, diabetes, as well as immune and inflammatory disorders. This review describes recent advances in understanding the molecular structure of UPR in mammalian cells, its functional role in cellular stress, and its pathophysiology.
Copyright © 2011 Wiley Periodicals, Inc.
Figures
References
-
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–95. - PubMed
-
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ice/ced-3 protease nomenclature. Cell. 1996;87:171. - PubMed
-
- Araki E, Oyadomari S, Mori M. Endoplasmic reticulum stress and diabetes mellitus. Intern Med. 2003;42:7–14. - PubMed
-
- Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, Croxton R, Krajewska M, Zapata JM, Kupiec-Weglinski JW, Farmer D, Reed JC. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:2809–14. - PMC - PubMed
-
- Bando Y, Tsukamoto Y, Katayama T, Ozawa K, Kitao Y, Hori O, Stern DM, Yamauchi A, Ogawa S. Orp150/hsp12a protects renal tubular epithelium from ischemia-induced cell death. FASEB J. 2004;18:1401–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

