Runx2 controls a feed-forward loop between androgen and prolactin-induced protein (PIP) in stimulating T47D cell proliferation
- PMID: 21809344
- PMCID: PMC3376385
- DOI: 10.1002/jcp.22966
Runx2 controls a feed-forward loop between androgen and prolactin-induced protein (PIP) in stimulating T47D cell proliferation
Abstract
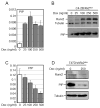
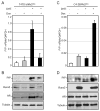
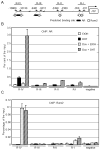
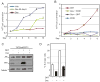
Prolactin-Induced Protein (PIP) is a small polypeptide expressed by breast and prostate cancer (BCa, PCa) cells. However, both the regulation of PIP expression and its function in cancer cells are poorly understood. Using BCa and PCa cells, we found that Runx2, a pro-metastatic transcription factor, functionally interacts with the Androgen Receptor (AR) to regulate PIP expression. Runx2 expression in C4-2B PCa cells synergized with AR to promote PIP expression, whereas its knockdown in T47D BCa cells abrogated basal as well as hormone stimulated PIP expression. Chromatin immunoprecipitation (ChIP) assays showed that Runx2 and AR co-occupied an enhancer element located ∼11 kb upstream of the PIP open reading frame, and that Runx2 facilitated AR recruitment to the enhancer. PIP knockdown in T47D cells compromised DHT-stimulated expression of multiple AR target genes including PSA, FKBP5, FASN, and SGK1. The inhibition of AR activity due to loss of PIP was attributable at least in part to abrogation of its nuclear translocation. PIP knockdown also suppressed T47D cell proliferation driven by either serum growth factors or dihydrotestosterone (DHT). Our data suggest that Runx2 controls a positive feedback loop between androgen signaling and PIP, and pharmacological inhibition of PIP may be useful to treat PIP positive tumors.
Copyright © 2011 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Akech J, Wixtted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, Stein GS, Lian JB. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2009;(29):811–821. - PMC - PubMed
-
- Bae SC, Choi JK. Tumor suppressor activity of RUNX3. Oncogene. 2004;23(24):4336–4340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

