Inhibin A enhances bone formation during distraction osteogenesis
- PMID: 21809377
- PMCID: PMC3737578
- DOI: 10.1002/jor.21501
Inhibin A enhances bone formation during distraction osteogenesis
Abstract
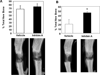
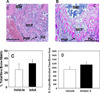
Given the aging population and the increased incidence of fracture in the elderly population, the need exists for agents that can enhance bone healing, particularly in situations of delayed fracture healing and/or non-union. Our previous studies demonstrated that overexpression of the gonadal peptide, human inhibin A (hInhA), in transgenic mice enhances bone formation and strength via increased osteoblast activity. We tested the hypothesis that hInhA can also exert anabolic effects in a murine model of distraction osteogenesis (DO), using both transgenic hInhA overexpression and administration of normal physiological levels of hInhA in adult male Swiss-Webster mice. Tibial osteotomies and external ring fixation were performed, followed by a 3-day latency period, 14-day distraction, and sacrifice on day 18. Supraphysiological levels of hInhA in transgenic mice, but not normal physiological levels of hInhA, significantly increased endosteal bone formation and mineralized bone area in the distraction gap, as determined by radiographic and µCT analysis. Significantly, increased PCNA and osteocalcin expression in the primary matrix front suggested that hInhA increased osteoblast proliferation. This mechanism is consistent with the effects of other agents and pathologies that modulate bone formation during DO, and demonstrates the potential of hInhA to enhance bone repair and regeneration.
Copyright © 2011 Orthopaedic Research Society.
Figures




Similar articles
-
The effect of aging on distraction osteogenesis in the rat.J Orthop Res. 2001 May;19(3):421-7. doi: 10.1016/S0736-0266(00)90025-1. J Orthop Res. 2001. PMID: 11398855
-
Distraction osteogenesis of the porcine mandible: histomorphometric evaluation of bone.Plast Reconstr Surg. 2004 Feb;113(2):566-73. doi: 10.1097/01.PRS.0000101061.99577.09. Plast Reconstr Surg. 2004. PMID: 14758219
-
Histomorphometry of distraction osteogenesis in a caprine tibial lengthening model.J Bone Miner Res. 1998 Jan;13(1):1-9. doi: 10.1359/jbmr.1998.13.1.1. J Bone Miner Res. 1998. PMID: 9443783
-
Biology of Bone Formation, Fracture Healing, and Distraction Osteogenesis.J Craniofac Surg. 2017 Jul;28(5):1380-1389. doi: 10.1097/SCS.0000000000003625. J Craniofac Surg. 2017. PMID: 28562424 Review.
-
[Bone fracture and the healing mechanisms. The mechanical stress for fracture healing in view of distraction osteogenesis].Clin Calcium. 2009 May;19(5):641-6. Clin Calcium. 2009. PMID: 19398830 Review. Japanese.
Cited by
-
Nutlin-3 treatment spares cisplatin-induced inhibition of bone healing while maintaining osteosarcoma toxicity.J Orthop Res. 2016 Oct;34(10):1716-1724. doi: 10.1002/jor.23192. Epub 2016 Feb 26. J Orthop Res. 2016. PMID: 26867804 Free PMC article.
-
Inhibition of GDF8 (Myostatin) accelerates bone regeneration in diabetes mellitus type 2.Sci Rep. 2017 Aug 29;7(1):9878. doi: 10.1038/s41598-017-10404-z. Sci Rep. 2017. PMID: 28852138 Free PMC article.
-
High-fat diet causes undesirable bone regeneration by altering the bone marrow environment in rats.Front Endocrinol (Lausanne). 2023 Mar 28;14:1088508. doi: 10.3389/fendo.2023.1088508. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37056669 Free PMC article.
-
Cisplatin inhibits bone healing during distraction osteogenesis.J Orthop Res. 2014 Mar;32(3):464-70. doi: 10.1002/jor.22527. Epub 2013 Nov 20. J Orthop Res. 2014. PMID: 24259375 Free PMC article.
-
Diabetes mellitus impairs bone regeneration and biomechanics.J Orthop Surg Res. 2023 Mar 6;18(1):169. doi: 10.1186/s13018-023-03644-5. J Orthop Surg Res. 2023. PMID: 36872328 Free PMC article.
References
-
- Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, Wark JD. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81:3366–3371. - PubMed
-
- Vural F, Vural B, Yucesoy I, Badur S. Ovarian aging and bone metabolism in menstruating women aged 35–50 years. Maturitas. 2005;52:147–153. - PubMed
-
- Guthrie JR, Ebeling PR, Hopper JL, Dennerstein L, Wark JD, Burger HG. Bone mineral density and hormone levels in menopausal Australian women. Gynecol Endocrinol. 1996;10:199–205. - PubMed
-
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M. FSH directly regulates bone mass. Cell. 2006;125:247–260. - PubMed
-
- Nicks KM, Fowler TW, Gaddy D. Reproductive hormones and bone. Curr Osteoporos Rep. 2010;8:60–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

