The inhibition of fat cell proliferation by n-3 fatty acids in dietary obese mice
- PMID: 21810216
- PMCID: PMC3162548
- DOI: 10.1186/1476-511X-10-128
The inhibition of fat cell proliferation by n-3 fatty acids in dietary obese mice
Abstract
Background: Long-chain n-3 polyunsaturated fatty acids (LC n-3 PUFA) of marine origin exert multiple beneficial effects on health. Our previous study in mice showed that reduction of adiposity by LC n-3 PUFA was associated with both, a shift in adipose tissue metabolism and a decrease in tissue cellularity. The aim of this study was to further characterize the effects of LC n-3 PUFA on fat cell proliferation and differentiation in obese mice.
Methods: A model of inducible and reversible lipoatrophy (aP2-Cre-ERT2 PPARγL2/L2 mice) was used, in which the death of mature adipocytes could be achieved by a selective ablation of peroxisome proliferator-activated receptor γ in response to i.p. injection of tamoxifen. Before the injection, obesity was induced in male mice by 8-week-feeding a corn oil-based high-fat diet (cHF) and, subsequently, mice were randomly assigned (day 0) to one of the following groups: (i) mice injected by corn-oil-vehicle only, i.e."control" mice, and fed cHF; (ii) mice injected by tamoxifen in corn oil, i.e. "mutant" mice, fed cHF; (iii) control mice fed cHF diet with15% of dietary lipids replaced by LC n-3 PUFA concentrate (cHF+F); and (iv) mutant mice fed cHF+F. Blood and tissue samples were collected at days 14 and 42.
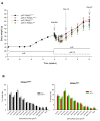
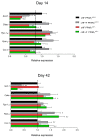
Results: Mutant mice achieved a maximum weight loss within 10 days post-injection, followed by a compensatory body weight gain, which was significantly faster in the cHF as compared with the cHF+F mutant mice. Also in control mice, body weight gain was depressed in response to dietary LC n-3 PUFA. At day 42, body weights in all groups stabilized, with no significant differences in adipocyte size between the groups, although body weight and adiposity was lower in the cHF+F as compared with the cHF mice, with a stronger effect in the mutant than in control mice. Gene expression analysis documented depression of adipocyte maturation during the reconstitution of adipose tissue in the cHF+F mutant mice.
Conclusion: Dietary LC n-3 PUFA could reduce both hypertrophy and hyperplasia of fat cells in vivo. Results are in agreement with the involvement of fat cell turnover in control of adiposity.
Figures
References
-
- Jelenik T, Rossmeisl M, Kuda O, Jilkova ZM, Medrikova D, Kus V, Hensler M, Janovska P, Miksik I, Baranowski M, Gorski J, Hebrand S, Jensen TE, Flachs P, Hawley S, Viollet B, Kopecky J. AMP-activated protein kinase α2 subunit is required for the preservation of hepatic insulin sensitivity by n-3 polyunsaturated fatty acids. Diabetes. 2010;59:2737–2746. doi: 10.2337/db09-1716. - DOI - PMC - PubMed
-
- Flachs P, Horakova O, Brauner P, Rossmeisl M, Pecina P, Franssen-van Hal NL, Ruzickova J, Sponarova J, Drahota Z, Vlcek C, Keijer J, Houstek J, Kopecky J. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia. 2005;48:2365–2375. doi: 10.1007/s00125-005-1944-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

