Temporal changes in HCV genotype distribution in three different high risk populations in San Francisco, California
- PMID: 21810243
- PMCID: PMC3199778
- DOI: 10.1186/1471-2334-11-208
Temporal changes in HCV genotype distribution in three different high risk populations in San Francisco, California
Abstract
Background: Hepatitis C virus (HCV) genotype (GT) has become an important measure in the diagnosis and monitoring of HCV infection treatment. In the United States (U.S.) HCV GT 1 is reported as the most common infecting GT among chronically infected patients. In Europe, however, recent studies have suggested that the epidemiology of HCV GTs is changing.
Methods: We assessed HCV GT distribution in 460 patients from three HCV-infected high risk populations in San Francisco, and examined patterns by birth cohort to assess temporal trends. Multiple logistic regression was used to assess factors independently associated with GT 1 infection compared to other GTs (2, 3, and 4).
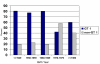
Results: Overall, GT 1 was predominant (72.4%), however younger injection drug users (IDU) had a lower proportion of GT 1 infections (54.7%) compared to older IDU and HIV-infected patients (80.5% and 76.6%, respectively). Analysis by birth cohort showed increasing proportions of non-GT 1 infections associated with year of birth: birth before 1970 was independently associated with higher adjusted odds of GT 1: AOR 2.03 (95% CI: 1.23, 3.34). African-Americans as compared to whites also had higher adjusted odds of GT 1 infection (AOR: 3.37; 95% CI: 1.89, 5.99).
Conclusions: Although, HCV GT 1 remains the most prevalent GT, especially among older groups, changes in GT distribution could have significant implications for how HCV might be controlled on a population level and treated on an individual level.
Figures
References
-
- Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, Halfon P, Inchauspe G, Kuiken C, Maertens G, Mizokami M, Murphy DG, Okamoto H, Pawlotsky JM, Penin F, Sablon E, Shin IT, Stuyver LJ, Thiel HJ, Viazov S, Weiner AJ, Widell A. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42(4):962–973. doi: 10.1002/hep.20819. - DOI - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965. doi: 10.1016/S0140-6736(01)06102-5. - DOI - PubMed
-
- McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group [see comments] N Engl J Med. 1998;339(21):1485–1492. doi: 10.1056/NEJM199811193392101. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- R01-DA021550/DA/NIDA NIH HHS/United States
- U19-AI40034-13/AI/NIAID NIH HHS/United States
- R01-DA016017-03A1/DA/NIDA NIH HHS/United States
- UL1-RR024131/RR/NCRR NIH HHS/United States
- K01-DA023365/DA/NIDA NIH HHS/United States
- R01-DA13245/DA/NIDA NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- P30 DK026743/DK/NIDDK NIH HHS/United States
- R01-DA09532/DA/NIDA NIH HHS/United States
- N01-CO-12400/CO/NCI NIH HHS/United States
- 2 R01 DA016017-03A1/DA/NIDA NIH HHS/United States
- P30-AI27763/AI/NIAID NIH HHS/United States
- R01-DA12109/DA/NIDA NIH HHS/United States
- R01 DA016017/DA/NIDA NIH HHS/United States
- H79-TI12103/TI/CSAT SAMHSA HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous