Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy
- PMID: 21810593
- PMCID: PMC3161308
- DOI: 10.2337/db10-1809
Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy
Abstract
Objective: The cannabinoid receptor type 2 (CB2) has protective effects in chronic degenerative diseases. Our aim was to assess the potential relevance of the CB2 receptor in both human and experimental diabetic nephropathy (DN).
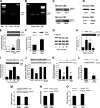
Research design and methods: CB2 expression was studied in kidney biopsies from patients with advanced DN, in early experimental diabetes, and in cultured podocytes. Levels of endocannabinoids and related enzymes were measured in the renal cortex from diabetic mice. To assess the functional role of CB2, streptozotocin-induced diabetic mice were treated for 14 weeks with AM1241, a selective CB2 agonist. In these animals, we studied albuminuria, renal function, expression of podocyte proteins (nephrin and zonula occludens-1), and markers of both fibrosis (fibronectin and transforming growth factor-β1) and inflammation (monocyte chemoattractant protein-1 [MCP-1], CC chemokine receptor 2 [CCR2], and monocyte markers). CB2 signaling was assessed in cultured podocytes.
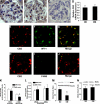
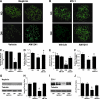
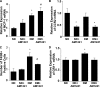
Results: Podocytes express the CB2 receptor both in vitro and in vivo. CB2 was downregulated in kidney biopsies from patients with advanced DN, and renal levels of the CB2 ligand 2-arachidonoylglycerol were reduced in diabetic mice, suggesting impaired CB2 regulation. In experimental diabetes, AM1241 ameliorated albuminuria, podocyte protein downregulation, and glomerular monocyte infiltration, without affecting early markers of fibrosis. In addition, AM1241 reduced CCR2 expression in both renal cortex and cultured podocytes, suggesting that CB2 activation may interfere with the deleterious effects of MCP-1 signaling.
Conclusions: The CB2 receptor is expressed by podocytes, and in experimental diabetes, CB2 activation ameliorates both albuminuria and podocyte protein loss, suggesting a protective effect of signaling through CB2 in DN.
Figures


References
-
- Molitch ME, DeFronzo RA, Franz MJ, et al. ; American Diabetes Association Nephropathy in diabetes. Diabetes Care 2004;27(Suppl. 1):S79–S83 - PubMed
-
- Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia 2001;44:1957–1972 - PubMed
-
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int 2006;69:73–80 - PubMed
-
- Giunti S, Barutta F, Perin PC, Gruden G. Targeting the MCP-1/CCR2 system in diabetic kidney disease. Curr Vasc Pharmacol 2010;8:849–860 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

