Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant: a 14-cohort meta-analysis
- PMID: 21810599
- PMCID: PMC3161318
- DOI: 10.2337/db11-0176
Total zinc intake may modify the glucose-raising effect of a zinc transporter (SLC30A8) variant: a 14-cohort meta-analysis
Abstract
Objective: Many genetic variants have been associated with glucose homeostasis and type 2 diabetes in genome-wide association studies. Zinc is an essential micronutrient that is important for β-cell function and glucose homeostasis. We tested the hypothesis that zinc intake could influence the glucose-raising effect of specific variants.
Research design and methods: We conducted a 14-cohort meta-analysis to assess the interaction of 20 genetic variants known to be related to glycemic traits and zinc metabolism with dietary zinc intake (food sources) and a 5-cohort meta-analysis to assess the interaction with total zinc intake (food sources and supplements) on fasting glucose levels among individuals of European ancestry without diabetes.
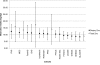
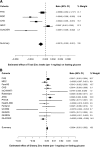
Results: We observed a significant association of total zinc intake with lower fasting glucose levels (β-coefficient ± SE per 1 mg/day of zinc intake: -0.0012 ± 0.0003 mmol/L, summary P value = 0.0003), while the association of dietary zinc intake was not significant. We identified a nominally significant interaction between total zinc intake and the SLC30A8 rs11558471 variant on fasting glucose levels (β-coefficient ± SE per A allele for 1 mg/day of greater total zinc intake: -0.0017 ± 0.0006 mmol/L, summary interaction P value = 0.005); this result suggests a stronger inverse association between total zinc intake and fasting glucose in individuals carrying the glucose-raising A allele compared with individuals who do not carry it. None of the other interaction tests were statistically significant.
Conclusions: Our results suggest that higher total zinc intake may attenuate the glucose-raising effect of the rs11558471 SLC30A8 (zinc transporter) variant. Our findings also support evidence for the association of higher total zinc intake with lower fasting glucose levels.
Figures


References
-
- Dupuis J, Langenberg C, Prokopenko I, et al. ; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC Investigators New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 - PMC - PubMed
-
- Staiger H, Machicao F, Fritsche A, Häring HU. Pathomechanisms of type 2 diabetes genes. Endocr Rev 2009;30:557–585 - PubMed
-
- Bantle JP, Wylie-Rosett J, Albright AL, et al. ; American Diabetes Association Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

