Osteopontin-stimulated expression of matrix metalloproteinase-9 causes cardiomyopathy in the mdx model of Duchenne muscular dystrophy
- PMID: 21810612
- PMCID: PMC3159792
- DOI: 10.4049/jimmunol.1101342
Osteopontin-stimulated expression of matrix metalloproteinase-9 causes cardiomyopathy in the mdx model of Duchenne muscular dystrophy
Abstract
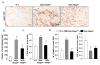
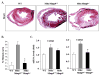
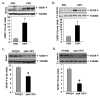
Duchenne muscular dystrophy (DMD), caused by mutations in the dystrophin gene, is a common and lethal form of muscular dystrophy. With progressive disease, most patients succumb to death from respiratory or heart failure, or both. However, the mechanisms, especially those governing cardiac inflammation and fibrosis in DMD, remain less understood. Matrix metalloproteinase (MMPs) are a group of extracellular matrix proteases involved in tissue remodeling in both physiologic and pathophysiologic conditions. Previous studies have shown that MMP-9 exacerbates myopathy in dystrophin-deficient mdx mice. However, the role and the mechanisms of action of MMP-9 in cardiac tissue and the biochemical mechanisms leading to increased levels of MMP-9 in mdx mice remain unknown. Our results demonstrate that the levels of MMP-9 are increased in the heart of mdx mice. Genetic ablation of MMP-9 attenuated cardiac injury, left ventricle dilation, and fibrosis in 1-y-old mdx mice. Echocardiography measurements showed improved heart function in Mmp9-deficient mdx mice. Deletion of the Mmp9 gene diminished the activation of ERK1/2 and Akt kinase in the heart of mdx mice. Ablation of MMP-9 also suppressed the expression of MMP-3 and MMP-12 in the heart of mdx mice. Finally, our experiments have revealed that osteopontin, an important immunomodulator, contributes to the increased amounts of MMP-9 in cardiac and skeletal muscle of mdx mice. This study provides a novel mechanism for development of cardiac dysfunction and suggests that MMP-9 and OPN are important therapeutic targets to mitigating cardiac abnormalities in patients with DMD.
Figures





Similar articles
-
Matrix metalloproteinase-9 inhibition ameliorates pathogenesis and improves skeletal muscle regeneration in muscular dystrophy.Hum Mol Genet. 2009 Jul 15;18(14):2584-98. doi: 10.1093/hmg/ddp191. Epub 2009 Apr 28. Hum Mol Genet. 2009. PMID: 19401296 Free PMC article.
-
Increased connective tissue growth factor associated with cardiac fibrosis in the mdx mouse model of dystrophic cardiomyopathy.Int J Exp Pathol. 2011 Feb;92(1):57-65. doi: 10.1111/j.1365-2613.2010.00750.x. Epub 2010 Dec 1. Int J Exp Pathol. 2011. PMID: 21121985 Free PMC article.
-
Matrix metalloproteinase-9 inhibition improves proliferation and engraftment of myogenic cells in dystrophic muscle of mdx mice.PLoS One. 2013 Aug 15;8(8):e72121. doi: 10.1371/journal.pone.0072121. eCollection 2013. PLoS One. 2013. PMID: 23977226 Free PMC article.
-
Proteomic profiling of the dystrophin-deficient mdx phenocopy of dystrophinopathy-associated cardiomyopathy.Biomed Res Int. 2014;2014:246195. doi: 10.1155/2014/246195. Epub 2014 Mar 20. Biomed Res Int. 2014. PMID: 24772416 Free PMC article. Review.
-
Modeling Duchenne Muscular Dystrophy Cardiomyopathy with Patients' Induced Pluripotent Stem-Cell-Derived Cardiomyocytes.Int J Mol Sci. 2023 May 12;24(10):8657. doi: 10.3390/ijms24108657. Int J Mol Sci. 2023. PMID: 37240001 Free PMC article. Review.
Cited by
-
Serum osteopontin, but not OPN gene polymorphism, is associated with LVH in essential hypertensive patients.J Mol Med (Berl). 2014 May;92(5):487-95. doi: 10.1007/s00109-013-1099-9. Epub 2013 Dec 27. J Mol Med (Berl). 2014. PMID: 24370940
-
Osteopontin stimulates matrix metalloproteinase expression through the nuclear factor-κB signaling pathway in rat temporomandibular joint and condylar chondrocytes.Am J Transl Res. 2017 Feb 15;9(2):316-329. eCollection 2017. Am J Transl Res. 2017. PMID: 28337262 Free PMC article.
-
COVID-19 Mimics Pulmonary Dysfunction in Muscular Dystrophy as a Post-Acute Syndrome in Patients.Int J Mol Sci. 2022 Dec 24;24(1):287. doi: 10.3390/ijms24010287. Int J Mol Sci. 2022. PMID: 36613731 Free PMC article. Review.
-
Deletion of Galgt2 (B4Galnt2) reduces muscle growth in response to acute injury and increases muscle inflammation and pathology in dystrophin-deficient mice.Am J Pathol. 2015 Oct;185(10):2668-84. doi: 10.1016/j.ajpath.2015.06.008. Am J Pathol. 2015. PMID: 26435413 Free PMC article.
-
Osteopontin regulates right ventricular failure through integrin ανβ3/PERK/CHOP-dependent inflammatory and apoptotic pathways.Front Immunol. 2025 May 6;16:1569210. doi: 10.3389/fimmu.2025.1569210. eCollection 2025. Front Immunol. 2025. PMID: 40396173 Free PMC article.
References
-
- Cox GF, Kunkel LM. Dystrophies and heart disease. Curr Opin Cardiol. 1997;12:329–343. - PubMed
-
- Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7:375–386. - PubMed
-
- Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–277. - PubMed
-
- Melacini P, Fanin M, Duggan DJ, Freda MP, Berardinelli A, Danieli GA, Barchitta A, Hoffman EP, Dalla Volta S, Angelini C. Heart involvement in muscular dystrophies due to sarcoglycan gene mutations. Muscle Nerve. 1999;22:473–479. - PubMed
-
- Melacini P, Gambino A, Caforio A, Barchitta A, Valente ML, Angelini A, Fanin M, Thiene G, Angelini C, Casarotto D, Danieli GA, Dalla-Volta S. Heart transplantation in patients with inherited myopathies associated with end-stage cardiomyopathy: molecular and biochemical defects on cardiac and skeletal muscle. Transplant Proc. 2001;33:1596–1599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

