Critical role for stromal interaction molecule 1 in cardiac hypertrophy
- PMID: 21810664
- PMCID: PMC3428713
- DOI: 10.1161/CIRCULATIONAHA.111.031229
Critical role for stromal interaction molecule 1 in cardiac hypertrophy
Abstract
Background: Cardiomyocytes use Ca2+ not only in excitation-contraction coupling but also as a signaling molecule promoting, for example, cardiac hypertrophy. It is largely unclear how Ca2+ triggers signaling in cardiomyocytes in the presence of the rapid and large Ca2+ fluctuations that occur during excitation-contraction coupling. A potential route is store-operated Ca2+ entry, a drug-inducible mechanism for Ca2+ signaling that requires stromal interaction molecule 1 (STIM1). Store-operated Ca2+ entry can also be induced in cardiomyocytes, which prompted us to study STIM1-dependent Ca2+ entry with respect to cardiac hypertrophy in vitro and in vivo.
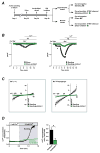
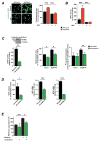
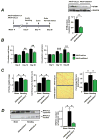
Methods and results: Consistent with earlier reports, we found drug-inducible store-operated Ca2+ entry in neonatal rat cardiomyocytes, which was dependent on STIM1. Although this STIM1-dependent, drug-inducible store-operated Ca2+ entry was only marginal in adult cardiomyocytes isolated from control hearts, it increased significantly in cardiomyocytes isolated from adult rats that had developed compensated cardiac hypertrophy after abdominal aortic banding. Moreover, we detected an inwardly rectifying current in hypertrophic cardiomyocytes that occurs under native conditions (i.e., in the absence of drug-induced store depletion) and is dependent on STIM1. By manipulating its expression, we found STIM1 to be both sufficient and necessary for cardiomyocyte hypertrophy in vitro and in the adult heart in vivo. Stim1 silencing by adeno-associated viruses of serotype 9-mediated gene transfer protected rats from pressure overload-induced cardiac hypertrophy.
Conclusion: By controlling a previously unrecognized sarcolemmal current, STIM1 promotes cardiac hypertrophy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Comment in
-
In vivo assessment of the risk profile of evolving individual coronary plaques: a step closer.Circulation. 2011 Aug 16;124(7):763-5. doi: 10.1161/CIRCULATIONAHA.111.042788. Circulation. 2011. PMID: 21844087 No abstract available.
-
Socking It to cardiac hypertrophy: STIM1-mediated Ca2+ entry in the cardiomyocyte.Circulation. 2011 Aug 16;124(7):766-8. doi: 10.1161/CIRCULATIONAHA.111.045179. Circulation. 2011. PMID: 21844088 Free PMC article. No abstract available.
References
-
- Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. - PubMed
-
- Guatimosim S, Dilly K, Santana LF, Saleet Jafri M, Sobie EA, Lederer WJ. Local Ca(2+) signaling and EC coupling in heart: Ca(2+) sparks and the regulation of the [Ca(2+)](i) transient. J Mol Cell Cardiol. 2002;34:941–950. - PubMed
-
- Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. - PubMed
-
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. - PubMed
-
- Molkentin JD. Calcineurin and beyond: cardiac hypertrophic signaling. Circ Res. 2000;87:731–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL078731/HL/NHLBI NIH HHS/United States
- R01HL093183/HL/NHLBI NIH HHS/United States
- K01 HL103176/HL/NHLBI NIH HHS/United States
- R01 HL088434/HL/NHLBI NIH HHS/United States
- R01HL083156/HL/NHLBI NIH HHS/United States
- R01HL088434/HL/NHLBI NIH HHS/United States
- P20HL100396/HL/NHLBI NIH HHS/United States
- R01 HL080498/HL/NHLBI NIH HHS/United States
- T32 HL007824/HL/NHLBI NIH HHS/United States
- R01HL078731/HL/NHLBI NIH HHS/United States
- P20 HL100396/HL/NHLBI NIH HHS/United States
- R01 HL083156/HL/NHLBI NIH HHS/United States
- R01HL080498/HL/NHLBI NIH HHS/United States
- R01 HL093183/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

