Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status
- PMID: 21810677
- PMCID: PMC3164246
- DOI: 10.1200/JCO.2010.34.4028
Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status
Abstract
Introduction: Retrospective studies suggest that p53 alteration is prognostic for recurrence in patients with urothelial bladder cancer and predictive for benefit from combination methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) adjuvant chemotherapy.
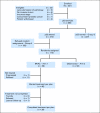
Patients and methods: Patients with pT1/T2N0M0 disease whose tumors demonstrated ≥ 10% nuclear reactivity on centrally performed immunohistochemistry for p53 were offered random assignment to three cycles of adjuvant MVAC versus observation; p53-negative patients were observed. By using a log-rank test with one-sided α = .05 and β = .10, 190 p53-positive patients were planned to be randomly assigned to detect an absolute improvement in probability of recurring by 3 years from 0.50 to 0.30.
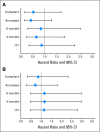
Results: A total of 521 patients were registered, 499 underwent p53 assessment, 272 (55%) were positive, and 114 (42%) were randomly assigned. Accrual was halted on the basis of the data and safety monitoring board review of a futility analysis. Overall 5-year probability of recurring was 0.20 (95% CI, 0.16 to 0.24) with no difference on the basis of p53 status. Only 67% of patients randomly assigned to MVAC received all three cycles with 12 patients receiving no treatment. There was no difference in recurrence in the randomly assigned patients (hazard ratio, 0.78; 95% CI, 0.29 to 2.08; P = .62).
Conclusion: Neither the prognostic value of p53 nor the benefit of MVAC chemotherapy in patients with p53-positive tumors was confirmed, but the high patient refusal rate, lower than expected event rate, and failures to receive assigned therapy severely compromised study power.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
p53 status in locally advanced bladder cancer.J Clin Oncol. 2012 Jan 20;30(3):339; author reply 339-40. doi: 10.1200/JCO.2011.39.0740. Epub 2011 Dec 19. J Clin Oncol. 2012. PMID: 22184388 No abstract available.
-
Words of wisdom. Re: Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status.Eur Urol. 2012 May;61(5):1062-3. doi: 10.1016/j.eururo.2012.02.011. Eur Urol. 2012. PMID: 22469412 No abstract available.
-
Re: Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status.J Urol. 2012 Jun;187(6):2024. doi: 10.1016/j.juro.2012.02.2550. Epub 2012 Apr 11. J Urol. 2012. PMID: 22579172 No abstract available.
-
Words of wisdom. Re: Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status.Eur Urol. 2012 Jul;62(1):183-4. doi: 10.1016/j.eururo.2012.04.014. Eur Urol. 2012. PMID: 22640860 Free PMC article. No abstract available.
References
-
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. - PubMed
-
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. - PubMed
-
- Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259–1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA027057/CA/NCI NIH HHS/United States
- U10 CA012644/CA/NCI NIH HHS/United States
- N01 CA032102/CA/NCI NIH HHS/United States
- U10 CA035192/CA/NCI NIH HHS/United States
- CA70903/CA/NCI NIH HHS/United States
- U10 CA014028/CA/NCI NIH HHS/United States
- R01 CA071921/CA/NCI NIH HHS/United States
- P30 CA014089/CA/NCI NIH HHS/United States
- U10 CA032102/CA/NCI NIH HHS/United States
- N01 CA027057/CA/NCI NIH HHS/United States
- CA71921/CA/NCI NIH HHS/United States
- U10 CA042777/CA/NCI NIH HHS/United States
- CA42777/CA/NCI NIH HHS/United States
- U10 CA046368/CA/NCI NIH HHS/United States
- N01 CA067575/CA/NCI NIH HHS/United States
- U10 CA035090/CA/NCI NIH HHS/United States
- CA12644/CA/NCI NIH HHS/United States
- CA14089/CA/NCI NIH HHS/United States
- CA14028/CA/NCI NIH HHS/United States
- CA58882/CA/NCI NIH HHS/United States
- CA35090/CA/NCI NIH HHS/United States
- CA46282/CA/NCI NIH HHS/United States
- U10 CA046282/CA/NCI NIH HHS/United States
- CA46368/CA/NCI NIH HHS/United States
- N01 CA038926/CA/NCI NIH HHS/United States
- U10 CA067575/CA/NCI NIH HHS/United States
- CA35192/CA/NCI NIH HHS/United States
- U10 CA058882/CA/NCI NIH HHS/United States
- U10 CA038926/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

