A cluster of ribosome synthesis factors regulate pre-rRNA folding and 5.8S rRNA maturation by the Rat1 exonuclease
- PMID: 21811236
- PMCID: PMC3209772
- DOI: 10.1038/emboj.2011.256
A cluster of ribosome synthesis factors regulate pre-rRNA folding and 5.8S rRNA maturation by the Rat1 exonuclease
Abstract
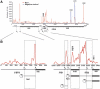
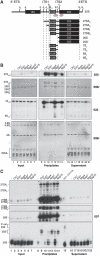
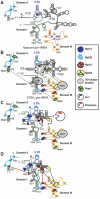
The 5'-exonuclease Rat1 degrades pre-rRNA spacer fragments and processes the 5'-ends of the 5.8S and 25S rRNAs. UV crosslinking revealed multiple Rat1-binding sites across the pre-rRNA, consistent with its known functions. The major 5.8S 5'-end is generated by Rat1 digestion of the internal transcribed spacer 1 (ITS1) spacer from cleavage site A(3). Processing from A(3) requires the 'A(3)-cluster' proteins, including Cic1, Erb1, Nop7, Nop12 and Nop15, which show interdependent pre-rRNA binding. Surprisingly, A(3)-cluster factors were not crosslinked close to site A(3), but bound sites around the 5.8S 3'- and 25S 5'-regions, which are base paired in mature ribosomes, and in the ITS2 spacer that separates these rRNAs. In contrast, Nop4, a protein required for endonucleolytic cleavage in ITS1, binds the pre-rRNA near the 5'-end of 5.8S. ITS2 was reported to undergo structural remodelling. In vivo chemical probing indicates that A(3)-cluster binding is required for this reorganization, potentially regulating the timing of processing. We predict that Nop4 and the A(3) cluster establish long-range interactions between the 5.8S and 25S rRNAs, which are subsequently maintained by ribosomal protein binding.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Ben-Shem A, Jenner L, Yusupova G, Yusupov M (2010) Crystal structure of the eukaryotic ribosome. Science 330: 1203–1209 - PubMed
-
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

